Download PDF
Ocular ischemic syndrome (OIS) comprises the ocular signs and symptoms attributable to chronic ocular hypoperfusion secondary to severe occlusion of the internal carotid artery. Ocular findings may be the only manifestation of carotid occlusive disease, although half of patients with OIS also have severe cardiovascular disease. Five-year mortality among patients with OIS is around 40%, with two-thirds of the deaths caused by myocardial infarction and one-third by stroke.1 Thus, timely diagnosis is necessary to reduce cardiovascular morbidity and mortality as well as to prevent permanent vision loss.
The estimated incidence of OIS is 7.5 cases per million persons every year; however, this figure may be an underestimate due to frequent misdiagnosis. OIS affects males twice as often as females because of the higher prevalence of cardiovascular disease in men, and it occurs at a mean age of 65 years.
OIS may be unilateral or bilateral. At the time of diagnosis, half of patients with OIS have complete obstruction of their ipsilateral internal carotid artery, and about 10% have complete obstruction bilaterally.2 Vascular risk factors include hypertension, diabetes, dyslipidemia, and heart disease.1 Other rare causes include giant cell arteritis and other inflammatory conditions, dissecting carotid aneurysm, Eisenmenger syndrome, and fibrovascular dysplasia.
Pathophysiology
The most common etiology is severe atherosclerosis-induced carotid stenosis resulting in greater than 90% occlusion of the ipsilateral carotid artery.2 Studies have shown that at 90% carotid occlusion, perfusion pressure of the ipsilateral central retinal artery is reduced by half. As a result of this ischemic insult, production of VEGF is increased, and anterior segment neovascularization develops in 66% of eyes.3 In rare cases, chronic obstruction of the ipsilateral ophthalmic artery or central retinal artery may be the cause.
 |
|
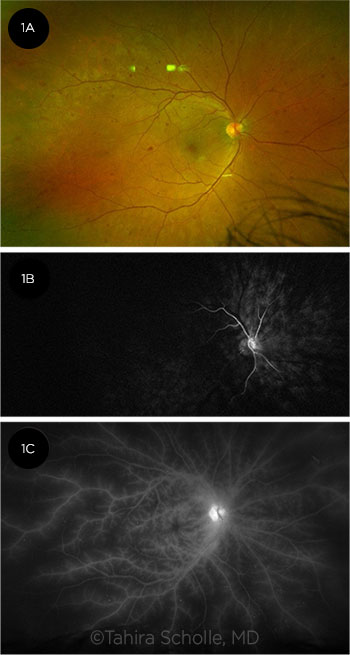
IMAGING OIS. (1A) Fundus photo shows deep, round, midperipheral intraretinal hemorrhages characteristic of OIS. FA demonstrates (1B) delayed choroidal and retinal filling at 28 seconds and (1C) late staining and leakage from retinal vessels due to endothelial ischemia.
|
Clinical Features
Symptoms. The most common symptom of OIS (in 67%-90% of cases) is gradual vision loss that develops over weeks to months.2 It is important to note that around 30% of eyes with OIS have visual acuity between 20/20 and 20/50 at presentation. However, studies confirm that once neovascularization of the iris (NVI) is present, 95% of eyes deteriorate to counting fingers vision or worse within one year.4
Other symptoms include amaurosis fugax; pain from elevated intraocular pressure (IOP) secondary to neovascular glaucoma (NVG); and prolonged visual recovery after exposure to bright light, a phenomenon caused by severe macular edema. Another manifestation is “ocular angina,” a dull, aching orbital pain of the affected eye due to ischemia of the globe or ipsilateral dura; the pain may be relieved when the patient is supine.3
Signs. Anterior segment signs include NVI (60%-66%) and anterior chamber cells or flare (20%-50%).2 Other findings include synechiae, hyphema, asymmetric cataract, conjunctival injection, and corneal edema.
These anterior segment signs result from impaired blood flow in the anterior and long and short posterior ciliary arteries. Approximately half of patients with OIS experience elevated IOP due to NVG, while the rest have normal or low IOP from impaired aqueous production.5
Posterior segment manifestations include narrowed retinal arteries (90%); dilated but not tortuous retinal veins (90%); retinal hemorrhages (80%); microaneurysms (80%); and, less frequently, cherry-red spot (12%); macular edema; or neovascularization of the optic nerve head (35%), retina (8%), or both.3 The retinal hemorrhages associated with OIS are typically round and deep and are most often located in the midperiphery, although they can also be found in the posterior pole (Fig. 1A).3
See “Signs of OIS” (below) for a more comprehensive list of anterior and posterior segment signs.
 |
|
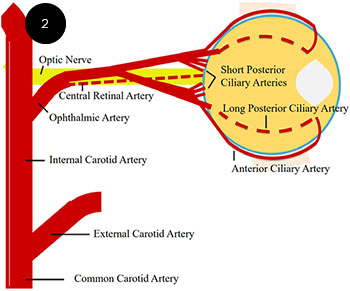
OCULAR BLOOD SUPPLY. OIS often causes ischemia of the long and short posterior ciliary arteries as well as the anterior ciliary arteries, leading to anterior segment manifestations.
|
Diagnosis
Carotid imaging. Carotid imaging is the most important modality to support a diagnosis of OIS. Carotid duplex ultrasound (DUS) is the most commonly performed initial diagnostic test, as it is accurate, noninvasive, low cost, and widely available.
Computed tomographic angiography or magnetic resonance angiography is frequently used as a confirmatory test or for surgical planning. Digital subtraction angiography is not used for initial diagnosis because it is invasive and carries a 1% to 2% risk of stroke, but it may be necessary if noninvasive tests show discordant results.
Although most patients with OIS have greater than 90% stenosis of the ipsilateral carotid artery, OIS can occur at lower levels of stenosis, depending on the patient’s collateral blood flow (or lack thereof). Therefore, carotid imaging showing greater than 90% stenosis strongly supports, but is not necessary to make, a diagnosis of OIS.
Ocular imaging. Ophthalmic imaging can assist the ophthalmologist with visualizing the extent of nonperfusion and subsequent damage to the iris, retina, and choroid. Features on fluorescein angiography (FA; see “Angiographic Findings,” below) include delayed choroidal filling and a prolonged arm-to-retina and arteriovenous transit time (Fig. 1B).3 Late arterial staining and leakage are also prominent due to chronic hypoxic endothelial ischemia (Fig. 1C). Indocyanine green angiography (ICGA) also shows prolongation of choroidal filling with diminished vascular filling in the midperiphery and posterior pole.
On OCT of the retina, patients with OIS were found to have a thinner choroid, indicating impaired choroidal circulation.6 OCT angiography has shown increased foveal avascular zone area and decreased retinal vessel density that improved after carotid artery reperfusion surgery.7
Other modalities. Several other modalities are less commonly used, but they may provide additional information.
- Electroretinography shows global amplitude reduction of both a-and b-waves due to poor perfusion to the inner and outer retinal layers caused by both retinal and choroidal vascular compromise.
- Visual evoked potential testing, not usually readily available in clinics, can show an increase in latency and prolonged recovery time after photostress.
- Ophthalmodynamometry or gentle palpation may be used to assess diminished central retinal artery perfusion, with easily collapsible vessels.3
Differential diagnosis. OIS is most frequently misdiagnosed as central retinal vein occlusion (CRVO) or diabetic retinopathy. Vision loss is typically acute in CRVO and chronic in diabetic retinopathy and OIS. In CRVO, the veins are both dilated and tortuous, unlike OIS, in which the veins are dilated but not tortuous. Dilation of veins is often attributed to an inflow obstruction, while the tortuosity seen in CRVO is chiefly due to an outflow obstruction.
Retinal hemorrhages are often present throughout the retina in CRVO and are most prominent in the posterior pole in diabetic retinopathy. In contrast, the retinal hemorrhages in OIS are most prominent in the midperiphery.
Because OIS causes ischemia of the long and short posterior ciliary arteries and of the anterior ciliary arteries (Fig. 2), it often leads to anterior segment ischemia. Therefore, signs of choroidal or anterior segment ischemia, such as anterior chamber cells and flare (and, rarely, keratic precipitates) or choroidal nonperfusion seen on FA or ICGA, may be present in OIS but not in CRVO or diabetic retinopathy.
Angiographic Findings
Prolonged retinal arteriovenous passage time (most sensitive)1
Prolonged choroidal filling time (most specific)1
Prolonged arm-to-retina time
Prolonged arm-to-choroid time
Prolonged retinal filling time
Staining of the major retinal vessels (mostly arteries) and their branches at the late phase
Foveal avascular zone enlargement
Capillary nonperfusion mainly in midperiphery
Late leakages of vessels and disc
Macular edema
___________________________
1 Brown GC, Magargal LE. Int Ophthalmol. 1988;11(4):239-251.
|
Management
Surgical management of carotid artery disease. The definitive treatment for OIS due to stenosis is carotid artery reperfusion surgery by means of carotid artery endarterectomy (CEA) or stenting.8 Thus, when the ophthalmologist suspects OIS, the patient should be referred to the primary care physician for appropriate coordination of care with the vascular surgery service.
Carotid artery reperfusion surgery has been shown to prevent the progression of chronic ocular ischemia as well as to reduce the risk of stroke. The North American Symptomatic Carotid Endarterectomy Trial demonstrated the superiority of CEA and aspirin therapy in preventing stroke compared with aspirin alone for both symptomatic and asymptomatic carotid artery stenosis. In addition, carotid reperfusion surgeries were shown to preserve visual acuity if performed before development of NVI, which is associated with a greater degree of ocular ischemia and damage. The American Academy of Neurology and the American Heart Association/American Stroke Association recommend CEA for symptomatic patients with 50% to 99% carotid artery stenosis and for asymptomatic patients with 60% to 99% stenosis.5
Carotid artery stenting and bypass surgery are alternative surgical options if CEA cannot be performed or if patients have medical contraindications.
In rare cases, carotid artery reperfusion has been shown to cause acute elevation of IOP due to increased aqueous formation and must be monitored and treated appropriately.5
Systemic risk reduction. In addition, all patients with suspected OIS should be referred to a primary care physician, cardiologist, or neurologist for evaluation and mitigation of cardiovascular risk factors. In patients with 100% stenosis, surgical treatment is ineffective; thus, medical management is especially important.
Ocular therapies. Topical steroids and cycloplegics can be used to manage anterior chamber reaction and to control pain. NVG may be treated medically or surgically. Cyclodestructive procedures may be needed to reduce IOP in a blind and painful eye.
Panretinal photocoagulation (PRP) can be performed for retinal neovascularization. However, regression of NVI occurred in only 36% of PRP-treated eyes with OIS, likely due to the severity of both retinal and choroidal ischemia.2
Intravitreal anti-VEGF agents such as bevacizumab have been used to successfully treat NVI, NVG, and macular edema secondary to OIS. However, the effect on visual acuity is unclear because patients’ vision is often limited by severe ischemia.9,10 Intravitreal triamcinolone acetonide can also be used to treat macular edema secondary to OIS, but it frequently requires multiple injections.5
Signs of OIS
|
ANTERIOR SEGMENT
|
POSTERIOR SEGMENT
|
|
More common:
Neovascularization of the iris (66%)
Anterior chamber cell and flare (20%-50%)
Conjunctival and episcleral injection
Sluggish light reflex
|
More common:
Dilated retinal veins (90%)
Narrowed retinal arteries (90%)
Retinal hemorrhages (usually midperipheral) (80%)
Microaneurysms (80%)
|
|
Less common:
Asymmetric cataract
Anterior and posterior synechiae
Atrophy of pupillary sphincter
Conjunctival chemosis
Corneal edema
Corneal epithelial erosion
Keratic precipitates
Corneoscleral melting
Hyphema Ectropion uveae
|
Less common:
Spontaneous retinal artery pulsations
Disc neovascularization
Retinal neovascularization
Cherry-red spot
Macular/disc edema
Glaucomatous nerve
Vitreous hemorrhage
Cotton-wool spots
Hard exudates
Anterior and posterior ischemic optic neuropathy
|
Conclusion
The ophthalmologist may be the first person to recognize carotid artery insufficiency because the signs and symptoms of OIS may manifest before other systemic signs. Thus, early recognition of OIS, based on clinical exam and supported by carotid imaging, is crucial in preventing blindness as well as in reducing cardiovascular morbidity and mortality.
The most important treatment for OIS is management of the underlying carotid occlusion through carotid reperfusion with CEA or stenting. Topical steroids and cycloplegics, PRP, intravitreal anti-VEGF, and intravitreal triamcinolone can be used to treat the local sequalae found in OIS. However, once NVI develops, the visual prognosis is poor, and vision is unlikely to improve.
___________________________
1 Sivalingham A et al. Int Ophthalmol. 1989; 13(3):187-191.
2 Terelak-Borys B et al. Med Sci Monit. 2012;18(8):RA138-RA144.
3 Brown GC, Magargal LE. Int Ophthalmol. 1988;11(4):239-251.
4 Malhotra R, Gregory-Evans K. Br J Ophthalmol. 2000;84(12):1428-1431.
5 Mendrinos E et al. Surv Ophthalmol. 2010;55(1):2-34.
6 Wang H et al. J Ophthalmol. 2017;2017:4169135.
7 Saito K et al. Retin Cases Brief Rep. Published online Feb. 4, 2019.
8 Lauria AL et al. Ann Vasc Surg. 2020;67:567.e9-567.e12.
9 Hazin R et al. Curr Opin Ophthalmol. 2009;20(6):430-433.
10 Amselem L et al. Am J Ophthalmol. 2007;144(1):122-124.
___________________________
Dr. Mohamed is a third-year ophthalmology resident, and Dr. Scholle is a retina specialist and assistant professor of ophthalmology. Both are at the Baylor College of Medicine, Houston. Financial disclosures: None.