By Rebecca Taylor, Contributing Writer, interviewing Joann A. Giaconi, MD, Ashish Sharma, MD, and Rishi P. Singh, MD
Download PDF
The diabetes drug semaglutide is back in the spotlight, thanks to its FDA approval this summer as a weight loss aid. As many ophthalmologists know, the drug has been associated with early worsening of diabetic retinopathy (DR). Given this expanded indication for treatment of obesity, do the benefits of semaglutide still outweigh the risks—and what should ophthalmologists expect?
Risk of DR Progession
Semaglutide is a glucagon-like peptide-1 (GLP-1) agonist, a metabolic hormone released by intestinal cells. It slows gastric emptying and reduces glucose absorption.1 “Semaglutide produces an impressive drop in blood sugar and hemoglobin A1c, so in patients for whom other medications cannot achieve control, it’s an important drug, because controlling blood sugar helps patients in the long run,” said JoAnn A. Giaconi, MD, at the Greater Los Angeles Veterans Administration and the Stein Eye Institute at the University of California, Los Angeles (UCLA).
A treatment paradox. However, the drug’s effectiveness presents clinicians with a challenge. As Dr. Giaconi said, “The VA in Los Angeles recently made a GLP-1 formulary switch to semaglutide, which is great for glycemic control, but it worsens existing diabetic retinopathy. I have clinicians asking me whether to switch patients back to their old diabetes drugs.”
This paradoxical worsening of DR after a sudden drop in blood glucose is a well-documented phenomenon. For instance, in a study of early worsening of DR after intensive insulin treatment, pancreas transplant, or bariatric surgery,2 10% to 20% of patients had worsening of DR within three to six months—with twice that many for patients who already had advanced DR at baseline.
And well-known trials such as the DCCT (Diabetes Control and Complications Trial) found an association between better blood glucose control and risk of early worsening of DR.
“It’s not uncommon to see this progression of DR if we improve glucose control,” said Rishi P. Singh, MD, at the Cole Eye Institute in Cleveland. “This happened back in the era of the DCCT, when patients had an intermittent worsening of retinopathy for the first year or two, but then had far better control of the retinopathy long-term.”
A question of heart versus eye. The global SUSTAIN trials (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes) demonstrated the drug’s gains in both A1c and weight loss.3 In SUSTAIN 6, a preapproval cardiovascular outcomes study, semaglutide showed an impressive 26% drop in cardiovascular events compared to placebo at two years.3 The study’s secondary endpoint, microvascular complications, turned up a lower risk of renal complications—but higher risk of retinal complications, such as early worsening of DR.
“They found DR progression in only the SUSTAIN 6 data,” said Ashish Sharma, MD, at Lotus Eye Hospital and Institute in Coimbatore, India. “SUSTAIN 1-5 excluded patients with known proliferative diabetic retinopathy, and the upper limit of A1c was 10 or 10.5, while SUSTAIN 6 had no exclusion criteria related to diabetic retinopathy and no upper limit of A1c.”
“The whole point with these drugs is that you’re trying to reduce mortality from cardiovascular risks,” said Dr. Singh. “Whether patients progress in diabetic retinopathy or not, I would rather we potentially ameliorate their macrovascular risk of cardiovascular and cerebrovascular diseases, instead of worrying about microvascular, eye-related risks that we can manage appropriately with current modalities.”
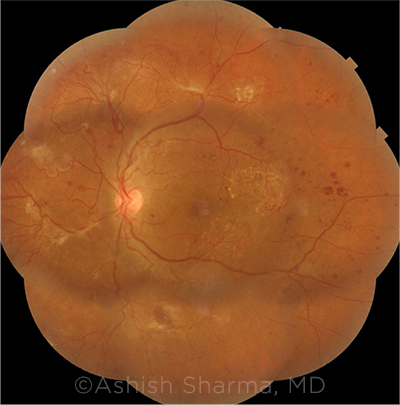 |
|
KEY QUESTION. Do the benefits of semaglutide still outweigh the risk of worsening of DR?Image courtesy of Ashish Sharma, MD, Lotus Eye Hospital and Institute, Coimbatore, Tamil Nadu, India.
|
New Indication, New Patients?
In June, the FDA approved a higher-dose formulation semaglutide for treatment of obesity,4 thanks in part to findings from the STEP (Semaglutide Treatment Effect in People) trials of people with obesity.
Eye-catching results. Results of STEP 1-4 were published this spring:
STEP 1 tested a 2.4 mg weekly injection of semaglutide plus lifestyle intervention in 1,961 patients without diabetes. The study found a drop in mean body weight of 14.9% at 68 weeks.5
STEP 2 evaluated 1,210 participants with type 2 diabetes and randomized them to receive either 1.0 mg semaglutide, 2.4 mg semaglutide, or placebo. At 68 weeks, average reduction in body weight was 9.6% with the higher dose, 6.99% with the lower dose, and 3.42% with placebo.6
STEP 3 assigned 611 participants to receive intensive behavioral therapy and either semaglutide 2.4 mg or placebo. After 68 weeks, 16% reduction in body weight was noted in those who had received the drug, versus 5.7% in the placebo cohort.7
STEP 4 evaluated semaglutide 2.4 mg in 902 participants, all of whom received the drug for 20 weeks and then were randomized to either placebo or semaglutide for an additional 48 weeks. On average, those who received the drug for the full 68 weeks lost 17.4% of their body weight—and those switched to placebo regained some of the weight they lost during the first phase, for a total loss of 5%.8
“We’ve never seen this kind of weight loss with a drug,” Dr. Sharma said. “Approximately one-third of patients lost more than 20% of body weight at 68 weeks of the STEP 2 trial, so it really is a breakthrough drug.”
Semaglutide at a Glance
|
Trade Name
|
Indication
|
Dosage
|
|
Ozempic
|
Type 2 diabetes
|
Once-weekly injection of 0.25 mg for initial four weeks and 0.5 mg for maintenance; may be increased to 1 mg if needed to maintain glycemic control.
|
|
Wegovy
|
BMI ≥30 or ≥27 in the presence of at least one weight-related comorbidity (e.g., hypertension, type 2 diabetes, or dyslipidemia).
|
Once-weekly injection of 2.4 mg.
|
|
BMI = body mass index
|
Level of Concern
Should ophthalmologists be concerned about an uptick of DR progression in a new cohort of patients?
“If a patient drops pounds by a significant amount, are we going to see a worsening of the retinopathy? That remains to be seen,” Dr. Singh said.
“I’m not too concerned, but I’m vigilant,” said Dr. Sharma. “Primary care physicians should be aware that this drug might cause DR progression, so we’re suggesting that before they start a patient on semaglutide, they should discuss it with an ophthalmologist.”
Need for caution. All three experts agree on the need for enhanced vigilance for patients on semaglutide, starting with a review of the patient’s systemic medications.
“The presence of semaglutide in a medical profile should alert the ophthalmologist to a potential risk,” said Dr. Singh. “But with good follow-up—and our ability to detect retinopathy and initiate treatment when necessary—this should not be a significant concern to many of the retina specialists taking care of these patients.”
Ideally, said Dr. Giaconi, primary care physicians should know the date of their patients’ last eye exam and, if DR was detected, its level of severity. “If the primary care doctor knows that a patient has severe nonproliferative DR, they may not want to start the patient on semaglutide,” she said. “Or if they do, the patient should have an eye exam around the time of drug initiation.”
She added, “The ophthalmologist’s responsibility is to know what diabetic medications the patient is on and if there’s been a recent switch. If your patient suddenly comes in on semaglutide, this person probably wasn’t under good glucose control and was switched to semaglutide” as a result.
Scheduling follow-up. Accelerated follow-up for patients starting on semaglutide depends on the severity of existing DR. “If patients have moderate retinopathy, instead of seeing them at six months, see them quarterly for awhile” to track retinopathy progression, said Dr. Giaconi. “If a patient has severe nonproliferative DR and you’d normally see them back in three months, this may now be someone you’ll see back in four to six weeks to make sure that no issues develop. For patients with mild retinopathy that you’d normally see yearly, consider checking them again in six months.”
Dr. Sharma added, “We don’t want to deprive patients of a drug that offers good glycemic control, weight reduction, and cardiac protection. But until I see the FOCUS trial results [see next page], I would be a little cautious, and I would recommend retinopathy screening before starting semaglutide.”
Monitoring progression. Drs. Giaconi and Singh noted that DR progression with semaglutide appears both temporary and manageable. “A worsening of diabetic retinopathy may occur, but things seem to stabilize after 12 to 18 months,” said Dr. Giaconi.
“In my opinion, the idea that patients are developing worsening retinopathy should not be a reason for patients to go off this drug,” said Dr. Singh. “I’m not concerned about progression rates because they have not been to the point where they’ve caused vision-threatening complications, and many cases are controllable now with our current therapies.”
Looking Ahead to FOCUS
The first trial to evaluate the long-term effects of semaglutide on DR in patients with type 2 diabetes is now recruiting participants.
The study, known as FOCUS, is enrolling 1,500 patients, who will be randomized to receive either placebo or semaglutide in addition to their diabetes medications. The primary outcome is progression of DR; secondary outcomes include the incidence of treatment with anti-VEGF injections, laser photocoagulation, or vitrectomy. The study is slated to conclude in February 2027.9
___________________________
1 Saw M et al. Eye (Lond). 2019;33(12):1842-1851.
2 Feldman-Billard S et al. Diabetes Metab. 2018;44(1):4-14.
3 Visbøll T et al. Diabetes Obes Metab. 2018;20(4):889-897.
4 www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014. Accessed Sept. 8, 2021.
5 Wilding JPH et al. New Engl J Med. 2021;384(11):989.
6 Davies M et al. Lancet. 2021;397(10278):971-984.
7 Wadden TA et al. JAMA. 2021;325(14):1403-1413.
8 Rubino D et al. JAMA. 2021;325(15):1414-1425.
9 https://clinicaltrials.gov; NCT03811561. Accessed Sept. 8, 2021.
___________________________
Dr. Giaconi is health sciences professor of ophthalmology at the Stein Eye Institute and David Geffen School of Medicine at UCLA. She also is chief of ophthalmology for the Greater Los Angeles Veterans Administration. Relevant financial disclosures: None.
Dr. Sharma is a vitreoretinal specialist at Lotus Eye Hospital and Institute in Coimbatore, India. Relevant financial disclosures: None.
Dr. Singh is a staff surgeon at the Cole Eye Institute and professor of ophthalmology at the Lerner College of Medicine at Case Western Reserve University in Cleveland. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Giaconi Allergan; C,L; MediPrint: C.
Dr. Sharma Allergan: C,L; Bayer: C,L; Intas: C,L; Novartis: C,L.
Dr. Singh Alcon: C; Apellis: S; AsclepiX: C; Bausch + Lomb: C; Carl Zeiss: C; Genentech: C; Gyroscope: C; NGM Biopharma: S; Novartis: C; Regeneron: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|