Download PDF
Public health officials and cornea specialists heralded the release of recombinant zoster vaccine (Shingrix, GlaxoSmithKline), given its ability to prevent herpes zoster (shingles) and ward off one of the disease’s most serious complications, herpes zoster ophthalmicus (HZO).
The second-generation vaccine for the prevention of shingles was licensed by the FDA for adults age 50 and older in October 2017. Since then, however, the vaccine supply has been plagued with shortages, and some patients have reported side effects that prevented them from following through with the two-dose protocol.
Although the shortages are expected to persist throughout this year,1 there is good news: According to the CDC, safety data for the eight months following FDA approval of Shingrix are consistent with comparable data from prelicensure clinical trials.2 “Systemic and local reactions were most commonly reported, but [they] tended to be nonserious and self-limited,” said lead author Elisabeth M. Hesse, MD, at the CDC in Atlanta.
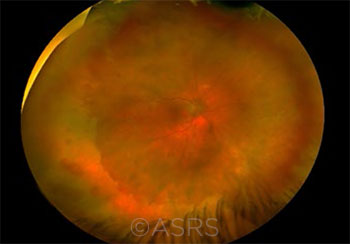 |
SHINGLES RISK. This 40-year-old woman presented with acute retinal necrosis due to HZO. This image was originally published in the ASRS Retina Image Bank. Olivia Rainey. Acute Retinal Necrosis Due to Herpes Zoster Ophthalmicus. Retina Image Bank. 2018; Image Number 27726. © The American Society of Retina Specialists.
|
Safety data. The postlicensure safety profile is based on reports to the Vaccine Adverse Event Reporting System (VAERS), a national passive surveillance system. VAERS received 4,381 reports of adverse events, including reports from health care providers and the public, between October and June 2018. During that time, some 3.2 million doses of Shingrix were distributed.
Adverse reactions. Signs and symptoms included the following:
- Chills, headache, fatigue, and myalgia were commonly reported, along with injection site reactions.
- Most common signs and symptoms included fever (23.6%), injection site pain (22.5%), and injection site erythema (20.1%).
- All told, 130 (3%) of events were classified as serious.
- People between the ages of 50 and 69 reported a high percentage of systemic signs and symptoms (e.g., chills and headache). In contrast, those age 70 and older reported a high frequency of local symptoms (e.g., injection site pain).
Reassurance. Overall, Dr. Hesse said, the CDC team was “reassured” by the findings. She added that providers should expect that some patients will experience reactions to the vaccine—but that most reactions will be self-limited and should resolve in a few days. The CDC and FDA will continue to monitor the vaccine’s safety profile, as the vaccine is still in the early uptake period.
Cornea risk reminder. Kathryn A. Colby, MD, PhD, at the University of Chicago, urged ophthalmologists to continue to educate patients that the vaccine is safe, effective, and can prevent HZO. “Herpes zoster ophthalmicus can cause serious cornea complications that can lead to permanent vision loss and chronic pain that impacts quality of life,” she said. “It’s good for ophthalmologists to educate patients on the benefit—because we’re the ones who will end up managing the complications. We need to get the word out.”
—Miriam Karmel
___________________________
1 CDC. Current vaccine shortages & delays. www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Updated November 2018. Accessed March 20, 2019.
2 Hesse EM et al. MMWR Morb Mortal Wkly Rep. 2019;68(4):91-94.
___________________________
Relevant financial disclosures—Drs. Colby and Hesse: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Colby WL Gore, Inc.: C.
Dr. Gupta None
Dr. Moore None
Dr. Hesse None
Dr. Rosen Astellas: C; Bayer: C; Boehringer Ingelheim: C; Diopsys: C; Genentech/Roche: C; Guardion Health: O; Nano-Retina: C; OD-OS: C; Opticology: O; Optovue: C; Regeneron: C; Teva: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review