Download PDF
Researchers at Emory University report a unique maculopathy associated with chronic exposure to pentosan polysulfate sodium (PPS), a drug approved by the FDA in 1996 to treat discomfort associated with interstitial cystitis (IC).1
Previously unreported. The previously unreported maculopathy is thought to primarily affect the retinal pigment epithelium (RPE). It may be mistaken for other well-known macular disorders such as pattern dystrophy or age-related macular degeneration (AMD).
“Hundreds, if not thousands, of patients diagnosed with pattern dystrophy and AMD since the drug’s approval may actually have a preventable drug-associated maculopathy,” said senior author Nieraj Jain, MD, at Emory Eye Center in Atlanta.
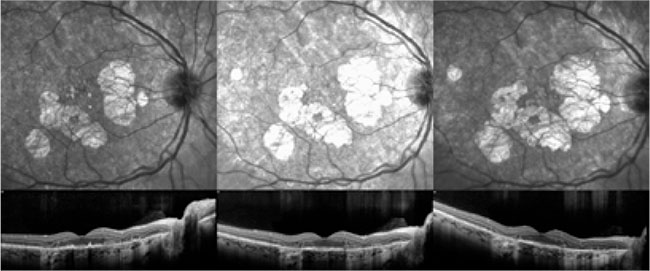 |
EVIDENCE. These images, taken over a two-year period in one patient, demonstrate the progressive nature of the patchy RPE atrophy noted in more severe cases of PPS-associated maculopathy.
|
Detective work. After seeing a string of patients with similar pigmentary macular changes and a past history of IC, the researchers culled their clinic’s electronic medical records for PPS. Within the prior two years, six patients had previously been identified by the authors for an unknown pigmentary maculopathy. “That makes it one of the more common conditions that we saw in our clinic of hereditary retinal diseases,” Dr. Jain said. (Since study publication in November 2018, the number of affected patients has grown to 15.)
Findings of note. The new entity mimics hereditary pattern dystrophies, yet none of the patients had a family history of hereditary retinal degeneration, and none showed a pathogenic genetic mutation. Findings on fundus autofluorescence imaging were quite prominent, yet the fundus exam revealed only subtle paracentral hyperpigmentation at the level of the RPE, with surrounding pale yellow deposits.
Median exposure to PPS was 186 months; most patients reported trouble reading and experienced prolonged dark adaptation despite generally well-preserved visual acuity.
Clinical implications. “PPS-associated maculopathy has a permanent spot on our differential diagnosis for atypical pigmentary maculopathies,” said lead author William Pearce, MD, at the Georgia Eye Institute in Savannah. “It is important that clinicians are aware of this association when evaluating patients with macular dystrophy or degeneration, as it could easily be overlooked due to the subtle findings.”
Looking ahead. Dr. Jain stressed that causality must be confirmed. Nevertheless, he advises his affected patients to stop taking PPS. Should a cause and effect be determined, was there anything about the drug-approval process that could have prevented this? “Probably not, given that these patients were on the drug for years before manifesting visual symptoms,” said Dr. Jain. “On the other hand, as pharmaceuticals become increasingly complex, we should recognize the vital role that clinicians play in the postmarket surveillance of novel therapies.”
—Miriam Karmel
___________________________
1 Pearce WA et al. Ophthalmology. 2018;125(11):1793-1802.
___________________________
Relevant financial disclosures—Drs. Jain and Pearce: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Jaffe AbbVie: C; Alcon: C; EyePoint Pharmaceuticals: C; Heidelberg Engineering: C; Neurotech USA: C; Novartis: C.
Dr. Jain None.
Dr. Merle Bausch + Lomb: C; Laboratoires Théa: S.
Dr. Mollan None.
Dr. Pearce None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review