Download PDF
Doctors at the University of Michigan Kellogg Eye Center have reported a series of 3 patients who developed uveal effusion syndrome following treatment with immune checkpoint inhibitors.1 This new immunotherapy for solid cancers may cause an autoimmune response that adversely affects various organ systems, including the eye.
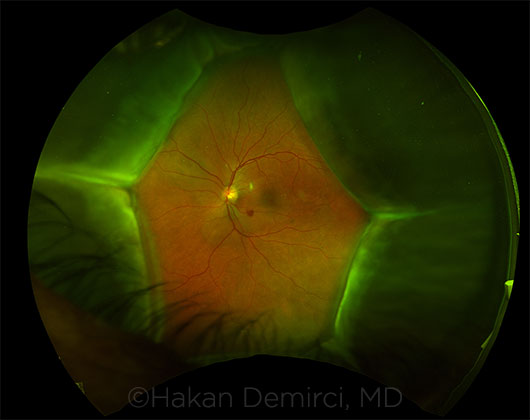 |
SIDE EFFECT. Choroidal effusions, observed during the fundus examination.
|
Ocular toxicity. When the 3 patients at the university-based ocular oncology clinic presented with uveal effusion, “the only recent change in their medical history was the start of the immune checkpoint inhibitor therapy,” said coauthor Hakan Demirci, MD, at the University of Michigan.
The men, ages 52 to 85, all developed uveal effusion 1 to 2 months after initiating therapy to treat either melanoma or lung cancer. Prior to presentation, each had received at least 2 infusions of the monoclonal antibodies atezolizumab, nivolumab, or pembrolizumab.
Symptoms and resolution. Anterior chamber inflammation was noted in 2 of the 3 cases, and visual acuity deteriorated and intraocular pressure spiked in all 3. The syndrome resolved after all immunotherapy was discontinued, without ophthalmic treatment, in 2 of the patients. In the third case, despite ocular issues, the patient continued therapy and died 4 months after initial presentation.
In the clinic. “We can’t advocate stopping the medication in these patients,” Dr. Demirci said. “This might not be possible because of the presence of the widespread metastatic disease.” He advised consulting the patient’s oncologist about systemic corticosteroid therapy to treat the eye. If that’s not possible, he recommended observing the patient to see if the uveal effusion worsens and affects vision.
The bottom line. Although the most common ocular complication of immune checkpoint inhibitors is uveitis (seen in about 0.3 to 0.6% of patients), ophthalmologists should be aware of this potential side effect, Dr. Demirci said. “Patients who present with uveal effusion should be questioned regarding the use of immune checkpoint inhibitors. Similarly, patients who use immune checkpoint inhibitors and who develop ocular symptoms should be evaluated for uveal effusion.”
—Miriam Karmel
___________________________
1 Thomas M et al. JAMA Ophthalmol. 2018;136(5):553-556.
___________________________
For more on ocular side effects of immune checkpoint inhibitors, see “Molecularly Targeted Cancer Drugs and Ocular Toxicity” in the October 2017 issue.
___________________________
Relevant financial disclosures—Dr. Demirci: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Connon Atelerix: O.
Dr. Demirci Castle Biosciences: C.
Dr. Fant Alcon: C; BSI: C; Clinical Research Consultants: E,O; CorNeat Vision: C; EyeYon Medical: C; HumanOptics: C; Oasis Medical: C; OptoQuest: C; PromiSight: E,O; Rashmivu: C; Reichert/Ametek: C; University of Louisville Coulter Foundation: C; University of Michigan Coulter Foundation: C; VEO Ophthalmics: E,O.
Dr. Greenberg None.
Dr. Snyder Alcon: S; Bausch + Lomb: S; Glaukos: S; Haag-Streit: C; HumanOptics: C,P; VEO Ophthalmics: O; W.L. Gore: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review