News in Review
Uveitis Guidelines: Immunomodulatory Therapy
Download PDF
Since guidelines for systemic treatment of noninfectious uveitis (NIU) were last published in 2000, treatment with biologic and other noncorticosteroid systemic immunomodulatory agents has become widespread. Now, an international, evidence-based consensus initiative has addressed the management of NIU in this new era of noncorticosteroid systemic immunomodulatory therapy (NCSIT).1
Rigorous methodology. Janet L. Davis, MD, MA, of the Bascom Palmer Eye Institute in Miami, emphasized the solid methodology behind the new recommendations. The group’s steering committee identified clinical questions, conducted a systematic review, and circulated proposed guidelines among 130 international uveitis experts. Group members met in late 2016 to refine guidelines in a modified Delphi technique and assign Oxford levels of evidence.
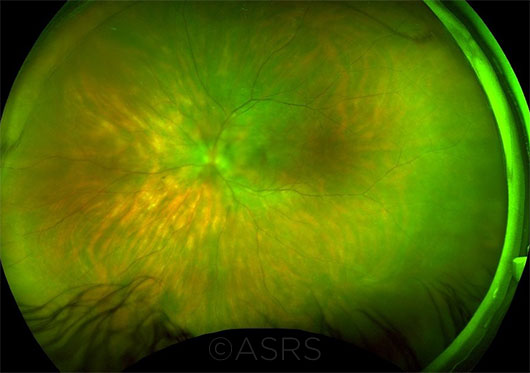 |
INDIVIDUALIZED TX. The guidelines provide recommendations by drug and disease. For instance, for birdshot chorioretinopathy (seen here), infliximab has a grade B recommendation, while intravenous immunoglobulins are grade C. This image was originally published in the ASRS Retina Image Bank. Armando L. Oliver, MD, and Carmen Santos, MD. Birdshot OS. Retina Image Bank. 2016; Image Number 26370. © The American Society of Retina Specialists.
|
Areas of clinical focus. The committee’s final guidance statements addressed optimal timing for treatment escalation; transitioning among agents, including biologics; and multidisciplinary team collaboration and safety monitoring.
Key guidelines. Dr. Davis encouraged ophthalmologists who manage uveitis patients to read the consensus guidelines, and she highlighted the following recommendations:
- NCSIT for NIU may be introduced to control persistent or severe inflammation or to prevent ocular structural complications that pose a risk to visual function (see Table 1).
- Collection of historical, laboratory, and clinically relevant radiologic data should take place before initiation of NCSIT. These data document baseline organ functions and test for active or latent infectious diseases.
- Although there is considerable heterogeneity in the criteria used to judge disease activity—cell counts; flare; haze; deterioration (or lack of response) in visual function; and retinal, choroidal, or optic nerve lesions—they can be influential in decisions to modify therapy.
- Before changing a therapy because of ineffectiveness, consider the following: treatment nonadherence, infections, and masquerade syndromes.
- If NCSIT is not adequately effective, escalation to the maximally tolerated dose may be considered before introducing an alternative medication, including a biologic agent (see Table 2). Choices for therapy must be individualized based on multiple factors, including the patient’s history, underlying cause of uveitis, and any systemic diseases.
- Withdrawal of NCSIT should be individualized based on tolerance of the current treatment, duration of disease control, and the specific cause of uveitis.
- Effective NCSIT drugs for NIU include mycophenolate mofetil (grade C recommendation), tacrolimus (grade B), cyclosporine (grade B), azathioprine (grade B), and methotrexate (grade B).
- Use of biologic agents for the treatment of NIU is supported for adalimumab (grade A recommendation), infliximab (grade B/C), and interferon alpha-2a (grade B).
- Communication across medical specialties, particularly between ophthalmologists and rheumatologists, fosters optimal therapy with safe prescribing and monitoring of NCSIT.
—Gabrielle Weiner
Table 1: Examples of Indications for Initiating Systemic Therapy
Ocular and anatomic
- Onset and course as defined by SUN Working Group criteria: acute disease that is sight threatening; chronic persistent inflammation
- Exudative retinal detachment
- Posterior and macular involvement
- Binocular sight-threatening disease
Therapeutic
- Regional failure: inadequate response to periocular steroid injections; inadequate response to topical corticosteroids in JIA-associated uveitis
- Systemic failure: active uveitis while taking doses of 30 mg or 0.5 mg/kg of prednisone per day or more; relapse of uveitis after reduction of the oral corticosteroid dose to less than 7-10 mg/day of prednisone; steroid intolerance; need for steroid-sparing effect
Severity
- In adults: VA worse than 20/100; increase in vitreous haze ≥ grade 2; relapse of cystoid macular edema; disease that impacts quality of life
- In JIA, severity includes prognostic factors for visual loss, such as: poorer presenting VA; posterior uveitis; uveitic complications of glaucoma; advanced cataract; macular edema; synechiae; severe band keratopathy; ocular hypotony; rubeosis iridis
JIA = juvenile idiopathic arthritis; SUN = Standardization of Uveitis; VA = visual acuity
Source: Adapted from Dick AD et al. Ophthalmology. Published online Jan. 6, 2018.
|
Table 2: When Response Is Inadequate
In any patient who is not benefiting adequately from NCSIT, the following steps are recommended.
Consider differential diagnosis.
Consider changing therapy, as follows:
- Dose escalation of current therapy
- Transition to alternative noncorticosteroid systemic agent
- Local or regional therapies
- Nonmedical therapy (vitrectomy, cryotherapy, etc.)
- Biologic therapy
Therapies should be individualized based on history, cause of uveitis, and patient preference.
Note: Currently, limited evidence exists to support adding an agent; safety and cost implications should be considered.
NCSIT = noncorticosteroid systemic immunomodulatory therapy
Source: Adapted from Dick AD et al. Ophthalmology. Published online Jan. 6, 2018.
|
___________________________
1 Dick AD et al., for the Fundamentals of Care for Uveitis International Consensus Group. Ophthalmology. Published online Jan. 6, 2018.
___________________________
Relevant financial disclosures: Dr. Davis—AbbVie: C; Allergan: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Bachmann Oculocare Medical: E,O.
Dr. Davis AbbVie: C; Allergan: C.
Dr. Doan NEI: S; Research to Prevent Blindness: S; Silicon Valley Community/Huang Pacific Foundation: S; UCSF Resource Allocation Program: S.
Dr. Gonzales NEI: S.
Dr. Marmor None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review