Download PDF
When a patient with an eye condition walks into an ophthalmologist’s office, the fact that she has been treated for breast cancer may not raise warning flags for the clinician. But there’s accumulating evidence that ocular conditions such as dry eye, retinopathy, and cataracts may be at least partly due to some breast cancer medications.
Only a small percentage of breast cancer patients experience clinically evident ocular side effects from their medications. Nevertheless, because these drugs are so widely used, the related eye conditions may affect many women. The breast cancer medication most commonly identified with ocular side effects is tamoxifen. However, chemotherapy agents, such as 5-fluorouracil (5-FU), can also have ocular side effects. And more researchers are becoming concerned that the drugs known as aromatase inhibitors, which now are often prescribed as adjuvant endocrine therapy, may also have adverse effects on the eye, including small retinal hemorrhages, increased incidence of floaters, and dry eye.
|
Tamoxifen Retinopathy
|
 |
|
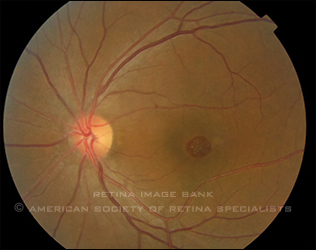
White or yellow refractile crystals around the macula are a characteristic finding in tamoxifen retinopathy (Image courtesty of Retina Image Bank. Young Hee Youn, MD; Sung Hyun Kim. 2012; #559. © American Society of Retina Specialists.)
|
Tamoxifen Troubles
“Tamoxifen has long been known to cause eye problems, including dryness, irritation, cataracts, and deposits in the retina, in the area of the macula, that result in macular edema,” said K. V. Chalam, MD, PhD, MBA, professor and chair of ophthalmology at the University of Florida College of Medicine in Jacksonville. Most of the ocular side effects are dose related, he said. “Certainly, the side effects we see with tamoxifen are much less profound than they used to be because of the lower doses now used.” Years ago, many breast cancer patients were prescribed doses of 150 mg or more, he noted. “In such cases, the ocular side effects from tamoxifen could be profound. That’s not the case anymore with the usual dose of 20 mg or less.”
Widespread effects. Tamoxifen is a selective estrogen receptor modulator (SERM) and acts against breast cancer by occupying estrogen receptors. It’s the only SERM approved for every stage of breast cancer. Because estrogen affects a wide variety of physiological functions and estrogen receptors are present in the eye, the changes in estrogenic activity brought about by tamoxifen have the potential to affect visual processing, as well as the lacrimal and meibomian glands that protect the surface of the eye, according to Alvin Eisner, PhD. Research shows that tamoxifen increases the risk of posterior subcapsular cataracts by as much as fourfold, which is significant because these types of cataracts can substantially impair visual function, said Dr. Eisner, a researcher most recently at the Northwest Sarcoma Foundation who specializes in ophthalmology, vision science, and cancer treatment.
Cataract. Researchers at the University of Southern California analyzed the self-reported incidence of eye disease among 1,297 female breast cancer patients taking tamoxifen who were enrolled in a population-based case-control study. They found that women who used tamoxifen for four to five years had a relative risk (RR) of 1.4 for all types of cataracts; those who were on the drug for more than six years had an RR of 1.7. They concluded that five or more years of tamoxifen increases cataract risk and that “healthy women considering tamoxifen use to reduce the risk of breast cancer should be advised of the possibility of cataract development.”1
Yet some ophthalmologists say that the question of whether tamoxifen increases risk of cataracts is still unresolved. “It’s fairly rare to find that a cataract is due to a medication, and I think that the jury is still out on whether tamoxifen may cause cataracts in some patients,” said Rick Fraunfelder, MD, MBA, professor of ophthalmology at Oregon Health & Science University and the Casey Eye Institute. Dr. Fraunfelder is also director of the National Registry of Drug-Induced Ocular Side Effects.
Retinopathy. However, use of tamoxifen, particularly at higher doses and for longer periods of time, may lead to retinopathy, Dr. Fraunfelder said. “Patients on tamoxifen can get striking white to yellow refractile bodies around the macula. These effects tend to occur at least one year after therapy begins and are cumulative.”
But, according to Dr. Eisner, tamoxifen retinopathy is overemphasized. A review article he coauthored states that “the initial findings of tamoxifen retinopathy at an auspicious time in the evolution of BC [breast cancer] treatment have led to an overemphasis on this condition in the sense that vision symptoms (e.g., photopsia) due to other intraocular conditions may be too readily misattributed to tamoxifen retinopathy and/or downplayed.”2 Estimates of the prevalence of retinopathy among breast cancer survivors on standard doses of tamoxifen have varied widely among published studies, with rates ranging from 0 to 6 percent, Dr. Eisner said.
Pointers for following patients on tamoxifen. Dr. Fraunfelder recommends that breast cancer patients have a baseline eye exam within the first year of treatment with tamoxifen, including an examination of the macula and testing of central and color vision. Dr. Chalam recommends that most breast cancer patients on tamoxifen be followed every four to six months, and those with symptoms should be seen by an ophthalmologist as often as every three months. Any sign of symptomatic ocular conditions should prompt a discussion with the patient as well as her oncologist.
According to Dr. Chalam, the ocular risks increase with long-term use of tamoxifen because the effects of the drug on the eye are cumulative over time. “The side effects usually occur at least one year after therapy begins,” Dr. Fraunfelder said. However, one condition that may occur relatively earlier is subclinical swelling within the optic nerve head, Dr. Eisner said. In addition, he noted that early, subtle cases of cystoid tamoxifen retinopathy may sometimes be detectable with optical coherence tomography (OCT).
The presence of asymptomatic refractile bodies is not a sufficient reason to discontinue tamoxifen, but if a patient starts to lose color vision or central vision while on the drug, the ophthalmologist should confer with the patient’s oncologist about stopping or switching treatments. Retinal hemorrhages and cystoid macular edema—which can result from tamoxifen use—may also indicate that a patient should stop taking tamoxifen or be switched to an alternative drug, Dr. Fraunfelder said.
The good news about the ocular side effects of tamoxifen is that if the drug is discontinued or the dosage reduced, ocular toxicities such as macular edema or retinal deposits are often reversible, Dr. Chalam said.
However, if the patient is on a high dose of tamoxifen and exhibits chronic maculopathy, there is a very real risk of losing vision permanently, Dr. Fraunfelder said. “It’s very rare for a patient to go completely blind, but many lines of visual acuity can be lost,” he said. “If you start getting vision loss after taking tamoxifen, it can be progressive and may continue even if you stop the drug.”
Alternatives to tamoxifen. Oncologists have the option of prescribing raloxifene (Evista) as an alternative to tamoxifen for breast cancer prophylaxis, and it may have a more favorable side effect profile. Although the National Surgical Adjuvant Breast and Bowel Project Study (STAR) found that there were fewer cases of breast cancer among tamoxifen users, women on raloxifene had fewer cases of uterine cancer, as well as of cataracts (RR = 0.79) and cataract surgeries (RR = 0.82).3
“Oncologists now have more choices, and patients experiencing ocular effects from tamoxifen can often be switched to raloxifene, although the use of raloxifene would be off label,” said Dr. Chalam.
Chemotherapy Complications
Chemotherapy drugs can also cause ocular side effects. According to Dr. Eisner, 5-FU can sometimes result in epiphora, while methotrexate can lead to conjunctivitis and other inflammation of the ocular surface.2
Because chemotherapies are toxic to rapidly dividing cells (such as those in a tumor), they can also be harmful to other cells that divide and renew on a regular basis, including those in the corneal epithelium. As a result, these agents can cause dry eye, which can usually be treated with lubricating drops, according to Dr. Chalam. Docetaxel, a taxane drug, can also lead to epiphora, as a result of stenosis of the tear drainage apparatus, said Dr. Eisner.
Conjunctivitis may occur when chemotherapy drugs leak into the tear film. “The chemicals irritate the eye, but the condition is temporary and goes away once the patient is done with chemotherapy,” Dr. Fraunfelder said, adding that there is no long-term damage. Topical nonsteroidal anti-inflammatory eyedrops can be used along with artificial tears if a patient has eye pain, he said.
Aromatase Inhibitors
In recent years, aromatase inhibitors (AIs) such as anastrozole (Arimidex), letrozole (Femara), and exemestane (Aromasin) have been increasingly prescribed to postmenopausal breast cancer patients as adjuvant endocrine therapy, sometimes after two to three years of tamoxifen treatment. The short-term ocular side effects of AIs often seem to be mild, at least according to the limited cross-sectional studies that have looked at this question, Dr. Eisner said, although “There is theoretical potential for AI-induced estrogen depletion to increase the long-term risk of serious eye disease.”
Although AIs work by inhibiting estrogen synthesis rather than by occupying estrogen receptors as tamoxifen does, some of the AI side effects are similar to those of tamoxifen, according to Dr. Eisner. The estrogen suppression that occurs with AIs might be regarded as causing an accelerated female aging that resembles an exaggerated menopause.
A study by Dr. Eisner and colleagues suggests that anastrozole can cause small retinal hemorrhages in some patients.4 He said that his research indicates that breast cancer patients who take anastrozole are more likely to have retinal hemorrhages than tamoxifen users. These hemorrhages may be the result of excessive traction on the retina, caused by estrogen depletion.5 Related effects, such as posterior vitreous detachments, may occur during the natural menopausal transition, he said. Dr. Eisner noted that it’s possible to assess the tractional effects of AIs through the use of OCT. Other possible effects of AIs include photopsia and increased incidence of floaters, as well as dry eye.
Dr. Eisner pointed to the need for more studies of the effects of AIs, particularly longitudinal studies to better document and help clarify their ocular effects.
“As many as 40 percent of women completely abandon their use of AIs before the prescribed time because they can’t tolerate the side effects. Although the ocular side effects may be less important or less compelling than well-known side effects such as arthralgia or hot flashes, they nevertheless may provide the straw that breaks the camel’s back for breast cancer patients on these drugs,” said Dr. Eisner.
___________________________
1 Paganini-Hill A, Clark LJ. Breast Cancer Res Treat. 2000;60(2):167-172.
2 Eisner A, Luoh SW. Curr Eye Res. 2011;36(10):867-885.
3 Vogel VG et al. JAMA. 2006;295(23):2727-2741.
4 Eisner A et al. Optom Vis Sci. 2008;85(5):301-308.
5 Eisner A et al. Breast Cancer Res Treat. 2009;117(1):9-16.
___________________________
Drs. Chalam and Eisner report no related financial interests. Dr. Fraunfelder is a consultant to Teva.