Download PDF
California scientists have developed a noninvasive method to detect nanometer-scale changes in the shape of human nerve cells as they fire, a development that could someday enable ophthalmic researchers to assess and quantify the eye’s neural functioning at the cellular level.
Using an interferometric microscope and a high-speed camera that imaged in vitro cells at up to 50,000 frames per second, the researchers assembled videos showing the membranes rounding slightly as they fired, then returning to normal.1
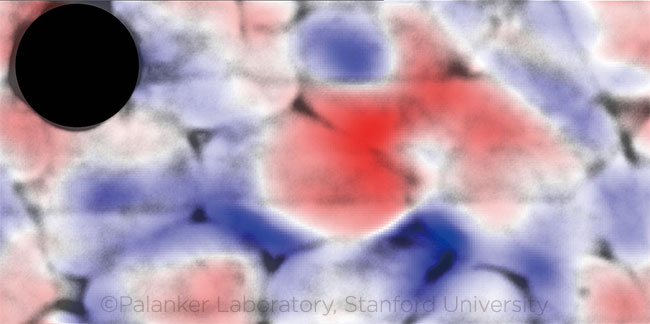 |
IN VITRO. Color overlay of firing nerve cells shows membrane deformation at the peak of the action potential. (Gray = nerve cells; red = movement toward viewer; blue = movement away from viewer; black dot = opaque electrode.) This image is licensed under a Creative Commons Attribution 4.0 International License and reproduced by permission of the authors. The image was adapted from the original version, which was published in Light: Science & Applications. 2018;7(107). To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0.
|
The genetically altered human cell line used in their experiments, called HEK-293, was chosen because it has regular, spontaneous electrical spikes. To separate the minuscule deformations from noise in the data, the scientists combined 50 frames at a time, averaged each pixel to strengthen the signal, and then used a self-reinforcing algorithm to boost the signal further.
In this way, they determined that the cells’ outer dimensions changed by between 1 and 3 nm, fluctuating as the action potentials propagated across the cells. These surrogate optical measurements of electrical activity corresponded precisely to the signals detected conventionally with electrodes placed near the cells.
“This nanometer-scale shape change is very difficult to see—but with ultra-fast quantitative phase imaging, it actually turns out to be visible,” said Daniel Palanker, PhD, who led the investigation.
Advantage: noninvasive. The technique’s major potential advantage compared to existing methods of measuring in vivo neuronal activity in the eye is that it is noncontact and noninvasive, said study coauthor Kevin C. Boyle, MS, a PhD student in Dr. Palanker’s laboratory at Stanford University in Palo Alto, California.
“Nothing needs to be added to the cells—no fluorescent dyes, no optogenetic viruses, no markers, no additional preparation. It’s all done optically,” Mr. Boyle said. “It’s also high throughput. You’re getting much more information about what’s happening across an individual cell and also across multiple cells in a field of view.”
Deformation of nerve cells when they fire was first described decades ago, based on observations of large nerves from crustaceans, he said. “But no one has been able to see the real thing in mammalian cells because the deformations are much smaller,” Mr. Boyle said.
But why do the membranes deform at all? “Based on our current hypothesis, which is from a model developed by others who have studied this effect, we believe that when the action potential happens the electrical potential generated across the cell membrane changes the membrane tension. This change tends to minimize the surface area of the cell membrane, causing the cell to become more spherical during the action potential,” he said.
Ultimate goal. The NEI views better imaging as essential for the advancement of regenerative therapies for retinal diseases, and it is funding five such projects through its Audacious Goals Initiative. This new technique, along with adaptive optics and optical coherence tomography, may eventually be used to build a device to noninvasively assess the electrical activity of the optic nerve and retinal cells.
—Linda Roach
___________________________
1 Ling T et al. Light Sci Appl. 2018;7:107.
___________________________
Relevant financial disclosures—Mr. Boyle and Dr. Palanker: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Asaoka Kowa: S; Oculus: S; Reichert Technologies: S.
Mr. Boyle None.
Dr. Hsu Genentech/Roche: S; Ophthotech: S; Santen: S.
Dr. Palanker DigiSight: C; Johnson & Johnson: C; Pixium Vision: C,P; Topcon Medical Systems: C,P.
Dr. Small None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review