Download PDF
Quick tips on imaging and diagnosis.
Is it wet AMD—or is it something else? “When a patient who is being treated for wet AMD isn’t responding as I expect, I have to ask that question,” said Darin R. Goldman, MD, at Retina Group of Florida, because it’s not uncommon to encounter patients with conditions that can be confused with neovascular AMD.
“Ophthalmologists should have a high index of suspicion for other entities, despite the fact that AMD is so common,” said K. Bailey Freund, MD, at Vitreous Retina Macula Consultants of New York. “Don’t assume that everything that has fluid and/or blood in the macula is wet AMD. Not all macular exudation is due to neovascular AMD. There are lots of other causes to consider.”
To examine the retina, ophthalmologists have an array of imaging modalities at their disposal: optical coherence tomography (OCT), fluorescein angiography (FA), indocyanine green angiography (ICGA), and fundus autofluorescence (FAF). It used to be that a single imaging modality was considered sufficient to view the retina, said Daniela Ferrara, MD, PhD, at Tufts University School of Medicine. Now we know that most of the retinal conditions are actually chorioretinal, and the primary site of injury may be at the level of RPE or the choriocapillaris. The various imaging techniques available today can get at these areas. “More and more, multimodal imaging gives you the best investigative strategy for both diagnosis and management of chorioretinal diseases,” she said.
“A lot of these are complicated, messy entities,” Dr. Freund said. Kavita V. Bhavsar, MD, at Moorfields Eye Hospital, agreed. “There are a lot of overlapping features between these diseases, but certain clinical features and multimodal imaging help clarify the correct diagnosis.”
Here is a concise guide to common conditions that may be confused with neovascular AMD.
Polypoidal Choroidal Vasculopathy (PCV)
It’s still debatable whether this variant of choroidal neovascularization (CNV) is a distinct entity or a subset of AMD, Dr. Ferrara said. Genetic information suggests the latter, since genes associated with AMD have been identified in PCV patients. But, she said, “It’s a gray area. Some people will say it’s a differential diagnosis, and some will say it’s within the spectrum of AMD.”
The primary PCV lesion is known to occur more often in specific demographic groups, such as Asian and African-American women. “Its lower incidence among Caucasians is one of the reasons why U.S. doctors do not routinely consider PCV when managing choroidal neovascularization with the clinical diagnosis of AMD,” Dr. Ferrara said.
CLINICAL CLUES. Clinical observations reveal polyp-shaped abnormalities in the vasculature thought to be located under the RPE, Dr. Goldman said. He noted that other features include the following:
- Multiple pigment epithelial detachments (PEDs).
- Atypical location of the lesion, either peripapillary or extramacular.
- Poor response to anti-VEGF treatment.
- Massive subretinal hemorrhage, which tends to occur more commonly in PCV than in AMD.
- Massive lipid deposition.
- Subfoveal choroidal thickness tends to be greater than normal in PCV, whereas the choroid is thinner than normal in AMD.
IMAGING. The experts discussed a range of modalities to help image PCV.
- ICGA is the so-called gold standard for imaging PCV. The polyps are commonly identified on ICGA, and this makes the diagnosis, said Dr. Goldman. Dr. Ferrara explained that indocyanine green is the only dye that can highlight the area beneath the RPE to show occult choroidal neovascularization.
Michael P. Kelly, an ophthalmic photographer at Duke Eye Center, advised that once PCV is found using ICGA, a simultaneous ICGA/OCT image should be performed to correlate structure and function.
- OCT shows all the abnormalities—irregular PEDs, subretinal fluid, and intraretinal exudates—but not as specifically as with ICGA. “OCT is supportive of a diagnosis, but not specific to it,” Dr. Goldman said. Dr. Ferrara noted that spectral-domain OCT (SD-OCT) may reveal the polypoidal lesion, if the examiner is looking for it. But she added that OCT is only as good as the scan. “You have to have the suspicion of PCV or be lucky to get the polyp on the spot,” she said. For best results, Mr. Kelly advised using SD-OCT 61-line volume scan “carpet bombing” of the macula and peripapillary region to ensure polyp capture.
- Specialized OCT. Enhanced depth imaging (EDI) OCT is useful for imaging the choroid, which can be thicker with PCV than with wet AMD. And, said Dr. Ferrara, newer swept-source OCT improves visualization of deeper tissue to better characterize the vascular structure of the choroid. She noted that swept-source en face OCT, which images deeper tissue and goes parallel to the tissue, might also capture polyps.
TREATMENT. Treatment is complicated by the fact that PCV has a different natural course than AMD. PCV can be self-limited and subside on its own, or it can progress, with dramatic diffuse subretinal bleeding, Dr. Ferrara said.
She starts with anti-VEGF therapy, but if the condition is still active, as demonstrated by continuing exudation or bleeding, she adds photodynamic therapy (PDT). “We know that for unresponsive cases, the combination may have higher efficacy for PCV than anti-VEGF alone,” she said.
In 2013, an expert PCV panel reported evidence-based guidelines for the diagnosis and treatment of PCV.1 Based on analysis of the literature, the results of the EVEREST trial, and their own experience, the panel recommended initial treatment of juxtafoveal and subfoveal PCV with ICGA-guided verteporfin PDT, or PDT plus 0.5-mg ranibizumab intravitreal injections one month apart for three months. If polyps don’t completely regress, then retreatment with PDT or PDT plus ranibizumab is called for. If ICGA shows complete regression of polyps, but other clinical anatomical signs of disease activity remain, and there’s leakage on FA, the authors advise re-treating with ranibizumab.
Dr. Bhavsar said, “Anti-VEGF therapy including ranibizumab and bevacizumab is typically successful in controlling PCV. However, in select refractory or progressive cases, aflibercept may be particularly successful and can be supplemented with PDT. My first-line agent for polypoidal disease is aflibercept.”
For more about PCV, read "Update on Polypoidal Choroidal Vasculopathy" in the December 2012 EyeNet.
Pearls From the Experts
DR. BHAVSAR. PCV and CSC are two conditions that are important to distinguish from neovascular AMD because they respond to therapy not typically employed for wet AMD, such as PDT.
DR. FREUND. Examine the other eye. If the fellow eye doesn't have any findings of macular degeneration, that's a clue that the condition is something other than AMD.
DR. GOLDMAN. My approach is, if it looks enough like macular degeneration, I'm going to probably treat it as such. But if the patient is not responding as I expect, I think: Could this be something else?
MR. KELLY. Communicate your clinical suspicion to the ophthalmic photographer, who will know the hallmarks and the specific features to document during imaging. Your imaging is only as good as your imager. |
Adult-Onset Vitelliform Macular Dystrophy (VMD)
Adult-onset VMD is a form of pattern dystrophy. “In my experience, this is the condition that’s most commonly misdiagnosed as wet AMD,” Dr. Goldman said. Multimodal imaging is critical for distinguishing this entity from neovascular AMD because anti-VEGF therapy is not effective for VMD, and proper diagnosis will spare the patient unnecessary treatment, he said.
CLINICAL CLUES. “Your clinical exam gives you a lot of information,” Dr. Goldman said. The typical presentation of a vitelliform lesion is a round collection of lipofuscin accumulation in the center of the macula that involves varying amounts of RPE atrophy. “This subretinal deposit can resemble the fluid in a choroidal neovascular membrane, but there’s never associated blood.”
IMAGING. Mr. Kelly said to consider the following:
- Color fundus photography to document circular foveal lesions
- FAF to show both hyper- and hypoautofluorescence in the macular region
- FA to show the mottled foveal hyperfluorescence with late staining
- OCT to show debris and a dome-shaped lesion (if present) in the macula
TREATMENT. None. Dr. Freund said that although subretinal fluid can appear and the angiogram can mimic the appearance of occult neovascular AMD, no neovascularization is present. Thus, there’s no role for anti-VEGF therapy.
Central Serous Chorioretinopathy (CSC)
CSC appears to be related to increased steroid levels, from either medications or endogenous sources such as stress, chronic disease, and pregnancy.
CLINICAL CLUES. This is a differential diagnosis of PCV, typically affecting younger patients. The primary manifestation involves subretinal fluid buildup, but if you see subretinal fluid in an older patient with multiple PEDs, you have to suspect AMD. The diagnosis becomes more complicated if CSC presents with secondary CNV—because once there is CNV, you can’t tell whether it’s secondary to CSC or if it’s PCV, Dr. Ferrara said.
IMAGING. A number of modalities can be useful for imaging CSC.
- FA is the primary modality, revealing diffuse RPE changes that extend beyond the macula. These may look like an ink spot or a smokestack, characteristic leakage patterns seen in this condition.
- OCT provides supportive evidence, Dr. Goldman said. It can reveal subretinal fluid with multiple PEDs, though the configuration is typically regular and circular. Caveat: “You have to look through the entire volume scan on OCT to identify the PEDs,” he said. Specialized OCT techniques can be used to obtain more informative images of the choroid, which is characteristically thick in CSC.
- ICGA localizes areas of leakage in the RPE.
- FAF reveals the extent of RPE changes, which are sometimes minimal on clinical examination. FAF is also valuable for diagnosis when it shows the typical “descending tract,” characteristic of the entity, Dr. Ferrara said.
TREATMENT. This is primarily a limited condition. Once the hypercortisolism (endogenous or exogenous) is controlled, the disease tends also to be controlled.
“In the absence of visual impairment, you are authorized to wait and observe for spontaneous resolution,” Dr. Ferrara said. If the patient cannot tolerate the visual impairment, if areas of retinal detachment are extensive and severe, or if symptoms persist for more than six months, the clinician should consider treatment.
The literature suggests that PDT may produce the best results. Several different protocols have been used: standard PDT protocol, low-fluence PDT with standard dose of verteporfin, or halfdose verteporfin with standard duration and power of PDT laser application.2 “The less aggressive protocols may have the best outcomes,” Dr. Ferrara said. Dr. Goldman added, “These patients do not respond well to anti-VEGF. Most cases will resolve in six months without any treatment. If it gets worse, PDT seems most useful.”
Pachychoroid Neovasculopathy
This is a newly defined condition falling within a spectrum of diseases, including CSC and PCV, that are associated with choroidal thickening (pachychoroid). “Certain patients with RPE changes due to an underlying pachychoroid appear susceptible to CNV as they age,” Dr. Bhavsar said.
Dr. Freund said this disease is subject to sub-RPE CNV, and it affects older people, but it’s not AMD. It has more in common with CSC than it does with AMD.
CLINICAL CLUES. Although this condition exhibits RPE abnormalities, a conspicuous lack of drusen and a thickened choroid are clues that it isn’t AMD, Dr. Bhavsar said.
IMAGING. Two types of imaging may be especially helpful in diagnosing pachychoroid neovasculopathy.
- EDI-OCT to visualize the choroid and to measure choroidal thickness. “If you want to image the choroid, you have to specifically ask the photographer to get an EDI scan,” Dr. Bhavsar said.
- FAF to reveal characteristic RPE changes.
TREATMENT. “I will usually try anti-VEGF first, but in treatment-resistant cases, I will add PDT,” Dr. Freund said.
Idiopathic CNV
By definition this is a CNV in which the cause is unknown. It primarily affects younger patients and may be associated with underlying inflammatory conditions. Although not common, it can occur in older patients. “Once it affects people over 50, it’s an obligatory differential diagnosis with AMD,” Dr. Ferrara said. Idiopathic CNV may be suspected when fundus features typical of AMD are absent, such as drusen. The differential diagnosis is clinical, and additional ancillary tests may not be necessary.
CLINICAL CLUES. Young age.
IMAGING. This condition looks like any other type of CNV.
- FA to identify CNV leakage
- OCT to provide supportive evidence of the characteristic subretinal and intraretinal fluid that is seen with CNV
TREATMENT. This condition typically runs a relatively limited course and usually responds well to anti-VEGF treatment, typically requiring fewer injections than AMD.3
Pathological Myopia
In the clinical setting, when patients older than 50 with high myopia develop CNV, the differential diagnosis between AMD and CNV secondary to myopia should be considered, Dr. Ferrara said.
CLINICAL CLUES. Clinical features such as lacquer cracks or Forster-Fuchs spot are classic examination findings that suggest CNV is secondary to pathological myopia, Dr. Bhavsar said.
IMAGING. Other modalities may be used in addition to fundus photography.
- EDI-OCT to view the choroid, which is significantly thinner in these patients than in healthy patients.4 Mirror artifact is often present in high myopes. If you encounter this, try vertical or oblique OCT scanning to flatten the scan, providing an interpretable image edge to edge, Mr. Kelly said.
- FA to identify the presence of neovascularization.
TREATMENT. The RADIANCE phase 3 randomized controlled study demonstrates the efficacy of ranibizumab 0.5 mg in improving and maintaining best-corrected visual acuity over a 12-month period.5
And a four-year retrospective study showed both ranibizumab and bevacizumab to be effective in treatment of pathological myopia.6
Vitreomacular Traction Syndrome (VMT)
“A clinical diagnosis is not useful for VMT. Our understanding of this disease has come full circle only with the advent of OCT,” Dr. Goldman said. VMT doesn’t usually mimic wet AMD, but it may occur concomitantly with AMD. In that case, the diagnosis of VMT may be overlooked or confounded with AMD.
CLINICAL CLUES. OCT reveals vitreous membranes inserting on and exerting traction on the macula. Other signs, such as cystic changes, schisis, and subretinal fluid, are nonspecific and may be seen in other disorders, including wet AMD, Dr. Goldman said.
IMAGING. Mr. Kelly advised using the following:
- OCT to document VMT and loss of foveal depression.
- OCT 24-line radial scanning to confirm that a small macular hole does not exist.
TREATMENT. The standard treatment is vitrectomy with peeling of the adherent membranes. Ocriplasmin, an intravitreal drug, was approved in 2012 for the treatment of symptomatic vitreomacular traction. However, ocriplasmin has limited efficacy and some remaining questions regarding safety, said Dr. Goldman.
A Complicated Diagnosis
When an older patient presents with CNV and subretinal hemorrhage, neovascular AMD is high on the differential diagnosis. Yet in one perplexing case where CNV was present, the diagnosis turned out to be central serous chorioretinopathy CSC. Multimodal imaging was key to making the diagnosis of CSC complicated by CNV, Dr. Bhavsar said.
CNV is fairly rare in CSC, but this patient had type 1 neovascularization, which was distinctly evident by EDI-OCT and ICGA.
EDI-OCT also revealed a substantially thickened choroid in both eyes. FAF revealed areas of previous gravitational detachment or tracts. Clinical observation revealed a suspicious paucity of high-risk soft drusen. Finally, the patient had a history of steroid use, which is a known significant risk factor for CSC.
The treatment for exudative complications from type 1 CNV in the setting of CSC is anti-VEGF therapy. This is very different from Dr. Bhavsar's management strategy for pure serous PED associated with more typical CSC, which can be observed.
If a patient with CSC has persistent and visually significant serous PED (without CNV), Dr. Bhavsar first considers PDT. "Anti-VEGF therapy has limited success in cases of pure serous PED lacking neovascularization, though some patients may respond to aflibercept. Notably, the combination of PDT and aflibercept may help maintain these patients in a fluid-free state," she said. |
Macular Telangiectasia
This is less commonly encountered than other AMD confounders, Dr. Goldman said. There are two types of macular telangiectasia. One, typically unilateral, is considered a variant of Coats disease. The second, usually bilateral, is more commonly confused with wet AMD.
CLINICAL CLUES. Cystic cavities in the temporal fovea on OCT, many small microaneurysmal abnormalities, and an absence of associated subretinal fluid.
IMAGING. Different stages of the disease may have different appearances, Dr. Goldman said.
- OCT reveals characteristic features, including lamellar defects within various layers of the macula. Typically, these appear in the temporal fovea rather than the nasal fovea. Over the fovea, there may also be an internal limiting membrane (ILM) drape related to underlying tissue loss. Mr. Kelly recommended a magnified high-resolution volume scan to reveal subtle early changes.
- FA reveals hyperfluorescent capillary dilatations with a parafoveal pattern that also favors the temporal fovea. Finely focused early FA images can define capillary dilatations.
- Confocal blue reflectance imaging. With critical focus, this may be useful to identify changes in the early states, Mr. Kelly said.
TREATMENT. “No treatment has been proven to be effective. However, if a secondary CNV is present, anti-VEGF therapy should be used,” said Dr. Goldman.
AND CONSIDER THE FOLLOWING
Dr. Freund notes several more conditions.
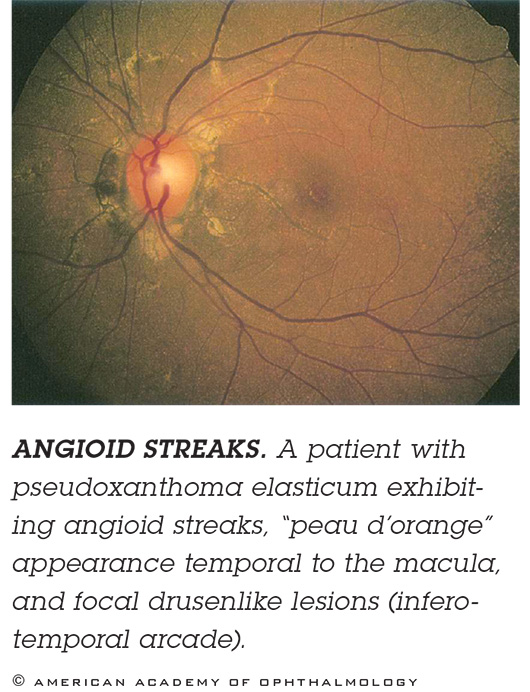
ANGIOID STREAKS. These breaks in Bruch’s membrane typically radiate out from the optic nerve and can be hard to see, so if you don’t look for them, you might mistakenly diagnose AMD. Patients with angioid streaks associated with pseudoxanthoma elasticum have a very high frequency of CNV. Imaging. FAF suffices, but ICGA is best. Treatment. CNV in these patients responds very well to anti-VEGF.
SMALL BRANCH RETINAL VEIN OCCLUSIONS. “Sometimes a small twig retinal vein occlusion (RVO) can masquerade as a type 3 neovascularization,” particularly in patients with drusen and RPE changes. Imaging. FA can be used to easily differentiate these two entities. Treatment. “While anti-VEGF is used to treat both wet AMD and RVO, an accurate diagnosis has important implications regarding patient counseling and prognosis for the fellow eye.”
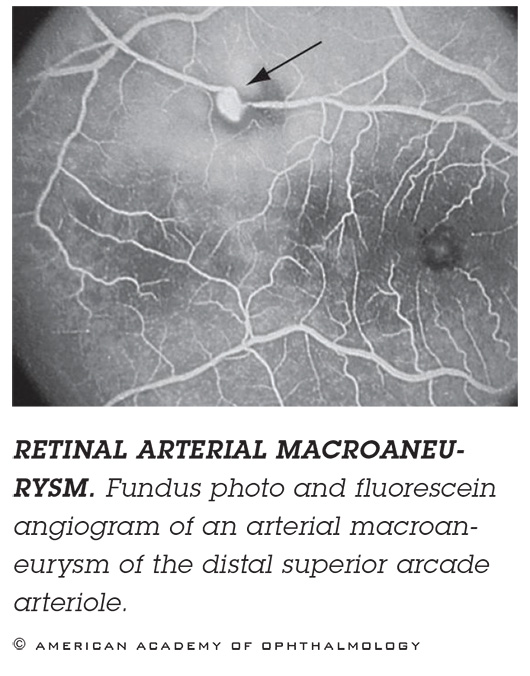
RETINAL ARTERIAL MACROANEURYSM. If you see blood, it may be hard to tell if it’s from a hemorrhage caused by wet AMD or from a ruptured macroaneurysm. Imaging. If it’s an aneurysm obscured by blood, ICGA might be useful. If there’s blood beneath the RPE, as revealed by OCT, then you’re dealing with AMD. Treatment. Observation or thermal laser.
__________________________
1 Koh AH et al. Retina. 2013;33(4):686-716.
2 Alkin Z et al. Clin Ophthalmol. 2014:8;685-690.
3 Zhang H et al. Am J Ophthalmol. 2012;153(2):300-306.
4 Ikuno Y et al. Invest Ophthalmol Vis Sci. 2010;51(7):3721-3725.
5 Wolf S et al. Ophthalmology. 2014;121(3):682-692.
6 Ruiz-Moreno JM et al. Br J Ophthalmol. 2013;97(11):1447-1450.
Meet the Experts
KAVITA V. BHAVSAR, MD Honorary fellow at Moorfields Eye Hospital. She has accepted a position as assistant professor in the retina division at Casey Eye Institute at the Oregon Health & Science University, in Portland. Disclosure: None.
DANIELA FERRARA MD, PHD Assistant professor of ophthalmology, Tufts University School of Medicine, in Boston. Disclosure: None.
K. BAILEY FREUND, MD In private practice at Vitreous Retina Macula Consultants of New York and clinical professor of ophthalmology, NYU School of Medicine, in New York City. Disclosure: Consults for Bayer Health Care, Genentech, Heidelberg Engineering, Regeneron, and ThromboGenics.
DARIN R. GOLDMAN, MD In private practice at Retina Group of Florida in Boca Raton. Disclosure: Consults for Bayer Health Care.
MICHAEL P. KELLY, FOPS Director of Duke Eye Imaging Labs, and Duke OCT Boot Camp at Duke Eye Center, Durham, NC. Disclosure: None. |