Download PDF
Better resolution. Higher scanning speeds. Eye-tracking capability. Despite major improvements in OCT technology, artifacts can still lead you—and your patients—down the wrong path. Learn tricks for avoiding the traps.
Even as technology advances, familiar problems may remain. Take, for instance, optical coherence tomography (OCT). Sequentially measuring reflections of laser light from the tissue of interest, time-domain OCT (TD-OCT) was limited by the speed of image acquisition and the number of images that could be obtained in the time a patient could sit still, said Sanjay G. Asrani, MD, glaucoma specialist at Duke Eye Center of Cary, N.C.
“Many of these challenges have been obviated by the advent of spectral-domain (SD) OCT,” he said, “which can produce significantly more detail in each image and allows detection of subtler changes in pathology at earlier stages.”
Faster acquisition speed of SD-OCT has minimized motion artifacts. And eye tracking features available with many commercially available devices also make it possible to follow the eye if it moves or blinks. Nevertheless, your evaluation of glaucoma or retinal diseases can be seriously compromised by motion artifacts, artifacts from operator or software error, or the presence of confounding pathology.
In fact, the overall artifact rate with SD-OCT is not very different from that of TD-OCT, said Glenn J. Jaffe, MD, retina specialist at Duke Eye Center in Durham, N.C.
Because artifacts haven’t gone away, and because they can interfere with interpretation of OCT images, it’s important to understand the problems that artifacts can pose, be familiar with the various types of artifacts and how to spot them, and know when to rescan the patient’s eye.
The Problem With Artifacts
“Interpreting OCT scans involves a combination of knowing whether the morphology is abnormal or normal, and whether the resolution is sufficient to identify problems,” said Dr. Jaffe. It’s also necessary to watch for artifacts for several important reasons.
They can compromise areas of interest. Artifacts become clinically significant when they can cloud clinical judgment due to incorrect interpretation, said Dr. Asrani. For example, said Dr. Jaffe, about 30 percent of the time, artifacts affect the center subfield—a circular region with a diameter of 1 mm centered on the fovea. This area is crucial for evaluating retinal diseases such as age-related macular degeneration (AMD) and diabetic macular edema.1
They can cause quantitative interference. Although artifacts may hinder qualitative comparisons of OCT images, they’re more likely to trip up the clinician’s quantitative analysis, said Jay S. Duker, MD, director of the New England Eye Center in Boston.
Misidentification of the retinal nerve fiber layer (RNFL) by the SDOCT software, for example, can lead to either a mistaken diagnosis of significant glaucoma, subjecting a patient to unnecessary testing or treatment, or a missed diagnosis, added Dr. Asrani.
They can act as confounders. A good example of a potentially confounding situation is that of uveitic glaucoma with inflammation-associated swelling in the RNFL: Is the thickness seen in the image due to edema or the tissue structure itself?2
“Swollen nerve fiber can fool a clinician into thinking there is no damage,” said Dr. Asrani. “Conversely, with uveitis control, a thinner RNFL than previously imaged may appear to be a sign of glaucoma progression. In addition, many glaucoma interventions involve procedures that result in subtle RNFL swelling, which can look deceptively like nerve preservation.”
They can mislead the unwary practitioner. Artifacts may interfere most when clinicians and researchers are assessing disease progression and treatment response, said Kaweh Mansouri, MD, MPH, glaucoma consultant, Geneva University Hospitals in Switzerland.
“Unless we are aware of how artifacts can mislead us, we can be led significantly astray,” said Dr. Asrani.
Types of Artifacts
There is currently no agreed-upon classification system for artifacts, said Dr. Mansouri. But for simplicity’s sake, he divides artifacts into categories of operator error and pathology.
Operator-related artifacts. “These play a big role but are largely ignored, simply because clinicians may not take the time or have the knowledge to look beyond the final printout, where not all artifacts are apparent,” said Dr. Mansouri. “Many operator errors can be corrected,” he said. “For example, you can manually readjust inaccurate segmentation lines or exclude B-scans with cut-edge artifacts.”
Common examples of operator-related artifacts include:
- Segmentation. With segmentation artifacts, the software doesn’t identify structures correctly and generates inaccurate numbers. This is the number-one artifact when evaluating retinal diseases, according to Dr. Duker, who said it occurs for reasons as diverse as dry eye, pathology, or motion during imaging. “Where operator error comes into play is when the interpreter doesn’t recognize the software failure and relies on the wrong information to make clinical decisions,” he said.
- Centration. The image is not centered in the grid used to calculate thickness of tissue. This is particularly important when monitoring thickness over time in response to therapy, said Dr. Jaffe.
- Motion. These types of artifacts are still a problem with SD-OCTs, especially with those that don’t include an eye-tracking system, said Dr. Duker. “Even though you acquire each individual scan more quickly with SDOCT than with TD-OCT, you’re still taking three seconds to do a macular scan, in which case a motion artifact can still occur.”
- Blink. When the patient blinks during scanning, blank areas are displayed by default in the en face images, and B-scans lose retinal data, said Dr. Mansouri.
- Cut edge. An edge of the image is cut off. These tend to be less problematic, said Dr. Jaffe, because, typically, they do not affect the central retinal thickness measurements and can often be eliminated by rescanning the eye. But they need to be recognized by the technician while the patient is still seated at the OCT machine. The technician can then promptly repeat the scan, which will usually solve the problem.
- Shadow. A variety of factors such as floaters can cast a shadow and result in a low signal, said Dr. Asrani.
- Mirror. The OCT generates two images, one a mirror image of the other. “Depending upon the placement of the scan, you may never see the mirror image,” said Dr. Jaffe. “But if the scan is not placed properly within the box, or if the person being scanned is very myopic (and the retina is very curved), you’ll see that mirror artifact.”3
Disease-state artifacts. With glaucoma, the most common artifact is coexisting pathology, said Dr. Asrani. For example, epiretinal membrane and vitreous traction can cause significant thinning or thickening of the RNFL and commonly affect glaucoma interpretation. However, the better resolution afforded by SD-OCT makes it possible to detect many of these artifacts more easily than in the past.4
Interpreting images based upon the classification in the OCT’s database can also lead to errors, said Dr. Asrani. These devices make calculations based upon a normative database of healthy subjects. “They usually don’t include conditions such as uveitis, AMD, high myopia, or diabetes,” added Dr. Mansouri. “Therefore these deviations can be erroneously flagged as glaucomatous.”
Red flags. It’s important, said Dr. Mansouri, to “have a heightened index of suspicion for artifacts whenever there’s a change or absence of change that’s contradictory to the rest of the ophthalmic examination.”
For example, be more skeptical about an OCT image that shows increased thinning if the IOP is well controlled, the optic nerve looks good, and visual fields are normal. “Don’t hesitate to go back to the technician and request to look at individual captured images,” he said.
Identifying Artifacts
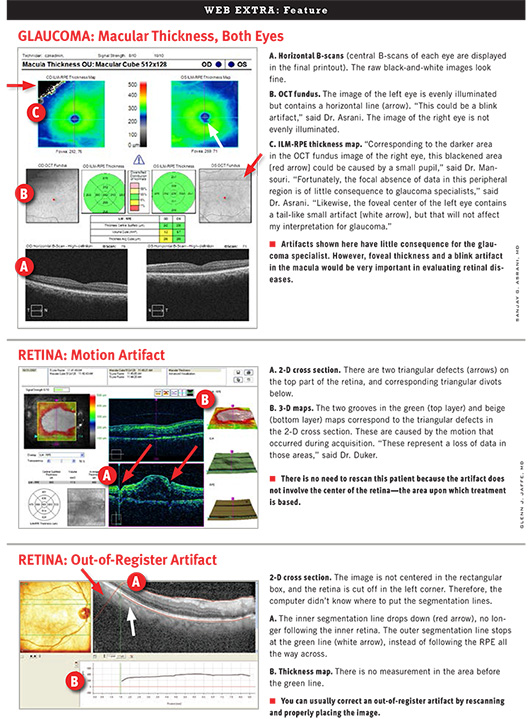
GLAUCOMA
Dr. Asrani and Dr. Mansouri recommend taking several steps to identify any artifacts in SD-OCT images when evaluating glaucoma.
1. Note whether signal strength is symmetrical between the two eyes, said Dr. Mansouri. To ensure quality resolution, make sure signal strength is at least 6 or greater in both eyes. Poor signal strength can be a major source of artifacts. For example, defocusing an image by at least 2 diopters can result in as much as a 10 µm thinning of the RNFL.5
2. Look at the qualitative information in the images.
- Check lines demarcating the boundaries of the RNFL to see whether the software correctly identified the RNFL.
- Check for lines or blacked-out areas; these are signs of a blink artifact.
- Check for irregular anatomic patterns such as a break in blood vessels, a sign of a motion artifact.
3. If you haven’t spotted any artifacts, you can review and rely upon quantitative data concerning the RNFL, optic nerve head, and ganglion cell loss. Parameters are represented as green, yellow, or red (from thickest to thinnest) or white for values outside of limits. “If artifacts are present, you will likely notice asymmetry or extreme values here,” said Dr. Mansouri. “In glaucoma, for example, the RNFL cannot logically be less than 40 microns because the glial cells themselves have a thickness of about 30 to 40 microns.”
Also, he said, “check to see how the OD and OS lines flow from temporal side to temporal side. Are they separate from each other in any location? Are they dipping into the red region in any one focal area?”
(click to expand)
Identifying Artifacts
RETINA
Dr. Jaffe and Dr. Duker recommend taking these three steps to identify artifacts in SD-OCT images when evaluating retinal diseases.
1. Look first at the color-coded macular map. The fovea should be centered. Check for areas of sudden change in the topography of the macula that can’t be explained by normal anatomy or pathology. For example, if you see triangular or square areas of blue (suggesting retinal thinning) within areas of white (extra thickness), you know that the software has failed. “It’s not physiologically possible to move from a thickened area of retina to a tiny area that’s really thin and back to a really thick area again,” said Dr. Duker.
2. To confirm the presence of an artifact, review the cross-sectional B-scans, which show where the software actually measured the thicknesses. Make sure the lines that the software drew correspond to what they should.
3. Know when the data are trustworthy. If artifacts are not present, you can review and rely upon the quantitative data.
(click to expand)
Scanning Protocols to Correct Artifacts
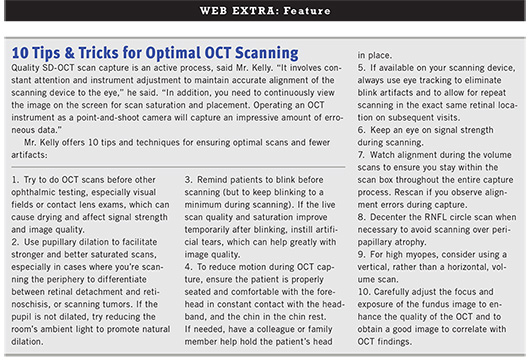
Michael P. Kelly, ophthalmic photographer and director of Duke Eye Imaging Labs at Duke Eye Center in Durham, N.C., noted several specific types of artifacts and recommended the following solutions.
Misidentification of the inner retina often occurs due to vitreomacular adhesion, vitreomacular traction, and vitreopapillary traction. Rescan using enhanced depth imaging, which reduces or eliminates the detail of the posterior vitreous.6 Or manually adjust the segmentation line after scanning.
Misidentification of the outer retina is often seen in patients with AMD, cystoid macular edema, and central serous retinopathy. Manually adjust the segmentation line after capture.
Eccentric fixation can affect the accuracy of macular thickness maps. Move the thickness map grid to the proper location after scanning. Or re-scan using the external fixation device, centering the fovea. For patients who can’t see either the internal or external fixation devices, put a Post-it with a big X on the wall for gross fixation, then swing/tilt the imaging head to center the fovea more accurately.
Mirror artifact may be encountered while scanning high myopes or elevated lesions such as tumors or retinal detachment, especially in the periphery. Try oblique or vertical scanning to eliminate or reduce the mirror artifact.
Iris vignetting can occur due to lack of dilation. Dilate the pupil, or turn off room lights and dim the monitor to allow for natural dilation.
Shadow artifact can be induced by factors such as a gas bubble, large floaters, or vitreous hemorrhage. Try to avoid the shadow by rescanning with oblique or vertical scan lines.
If a vitreous hemorrhage or large floater is obscuring the posterior pole, have the patient look away for a few seconds and then quickly back to the fixation point. This allows a small window of time for scan capture by temporarily shifting the hemorrhage and revealing the posterior pole.
__________________________
1 Han I et al. Ophthalmology. 2010;117(6):1177-1189.
2 Asrani S et al. JAMA Ophthalmol. 2014;132(7):877-880.
3 Ho J et al. Invest Ophthalmol Vis Sci. 2010;51(7):3714-3720.
4 Asrani S et al. JAMA Ophthalmol. 2014;132(4):396-402.
5 Balasubramanian M et al. Opt Express. 2009;17(5):4019-4036.
6 Mansouri K et al. Am J Ophthalmol. 2014;157(5):1022-1032.
Meet the Experts
SANJAY G. ASRANI, MD Clinical director, Duke Eye Center of Cary; professor of ophthalmology, Duke University School of Medicine, Durham, N.C. Financial disclosure: Consults for Dose Medical and Sucampo and receives lecture fees from Alcon, Heidelberg, and Merck.
JAY S. DUKER, MD Director, New England Eye Center; chairman and professor of ophthalmology, Tufts University School of Medicine, Boston. Financial disclosure: Consults and receives research support from Carl Zeiss Meditec; receives research support from Optovue; consults for Alcon/Novartis, Allergan, Nicox, Optos, Regeneron, Thrombogenics; and is a stockholder for Hemera Biosciences, EyeNetra, and Ophthotech.
GLENN J. JAFFE, MD Chief, Vitreoretinal Service, professor of ophthalmology, director, Duke Reading Center, Duke University School of Medicine, Durham, N.C. Financial disclosure: Consults for Heidelberg Engineering.
MICHAEL P. KELLY, FOPS Director, Duke Eye Imaging Labs and the Duke OCT BootCamp at Duke Eye Center, Durham, N.C. Financial disclosure: None.
KAWEH MANSOURI, MD, MPH Glaucoma consultant, department of ophthalmology, Geneva University Hospitals, Geneva, Switzerland; adjoint associate professor, University of Colorado, Denver. Financial disclosure: Consults for Sensimed and receives grant support from Sensimed and Topcon Japan. |