By Omar Moinuddin, MD, Thomas J. Wubben, MD, PhD, David N. Zacks, MD, PhD, and Cagri G. Besirli, MD, PhD
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
First described in 1986 by Hilton and Grizzard,1 pneumatic retinopexy (PR) is a nonincisional outpatient procedure used to treat selected cases of rhegmatogenous retinal detachment (RRD). PR is used to treat up to 15% of all retinal detachments in the United States, and it remains the most commonly employed modality for repair after pars plana vitrectomy (PPV) alone or PPV in combination with scleral buckle (SB).
PR involves the injection of an intravitreal gas or air bubble to tamponade the retinal break(s), coupled with laser retinopexy or cryoretinopexy to seal the break site(s). This two-step procedure facilitates apposition of the retina by means of the eye’s innate ability to resorb subretinal fluid (Fig. 1).
Indications
The ideal candidate for PR is phakic, with a single break or multiple smaller breaks spanning no more than 1 clock-hour in the superior 8 clock-hours of the fundus (Table 1). Relatively clear ocular media are necessary for the identification and treatment of the retinal break(s) that precipitated the RRD as well as other potential breaks in the retinal periphery.
The patient’s overall physical and cognitive health, as well as social environment and lifestyle, should allow for postprocedural head positioning such that the injected gas bubble remains over the retinal break(s).
Expanded criteria. PR has also been used successfully under expanded criteria to treat large retinal breaks, as well as several smaller breaks cumulatively spanning multiple clock-hours of the retinal arc.2,3 However, sequential alternation of head positioning may be required during the postoperative period to tamponade all retinal breaks effectively.4 For this reason, some surgeons choose to perform primary PPV, SB, or combined SB/PPV instead of PR to improve the likelihood of single-procedure success in patients with large tears or with several smaller breaks collectively spanning more than 1 clock-hour of the superior fundus.
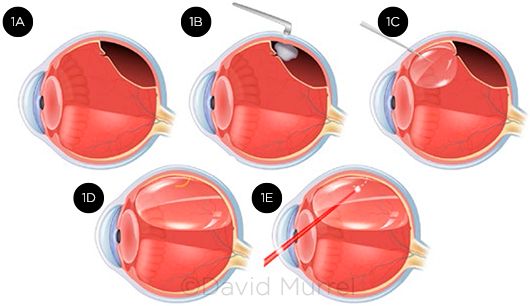 |
|
STEPS IN PR. (1A) Small retinal break allows fluid to enter the subretinal space, causing superior retinal detachment. (1B) Cryoretinopexy is used to stimulate scar formation around the edges of the break. (1C) Gas bubble is injected into the vitreous cavity. (1D) Bubble expands to cover and tamponade the retinal break. (1E) As an alternative to cryoretinopexy, laser photocoagulation can be performed around the retinal break after gas has been injected and retinal apposition is achieved.
|
Contraindications
Inferior break. Although single-operation and final anatomic success in the repair of inferior RRD has been reported in the literature,5 an inferior break is a general contraindication to PR. Even under maximal intravitreal expansion, the gas bubble may not cover the inferior retina with standard post-PR positioning. Furthermore, most patients cannot reasonably be expected to tolerate the inverted neck hyperextension or hyperflexion positioning required to tamponade an inferior break. Even in the management of an uncomplicated superior retinal break, physical or other disabilities that preclude appropriate head positioning may lead the physician to elect for SB, PPV, or both instead of PR.
Advanced glaucoma. Despite the use of anterior chamber paracentesis as part of the procedure, there is a risk for a spike in IOP with injection of gas into the vitreous cavity.4 Thus, advanced glaucoma may be a relative contraindication to PR. In all cases, after gas injection, central retinal artery perfusion should be confirmed by means of indirect ophthalmoscopy to visualize arterial pulsations. If pulsations are absent for more than 10 minutes, repeat paracentesis should be performed immediately to lower IOP.4
Proliferative vitreoretinopathy (PVR). Preexisting PVR with retinal traction may result in persistent RD despite adequate PR gas tamponade of the causative break.6 Thus, a patient with extensive PVR (grade C or D) is not a good candidate for PR.
Lens status. PR can be performed successfully in most phakic and pseudophakic patients. However, it should be avoided in patients with lens instability or aphakia because of the potential for gas bubble migration into the anterior chamber and poor tamponade of the retinal break(s).
Lattice degeneration. The presence of detachment or subretinal fluid accumulation itself is not a contraindication; surgeons may elect to perform PR if all breaks can be identified in the superior 8 clock-hours of the fundus and treated with laser or cryoretinopexy. Extensive lattice degeneration, however, may represent an increased risk for new retinal breaks and is considered a contraindication by some surgeons.3
Table 1: Indications and Contraindications
|
| INDICATIONS |
CONTRAINDICATIONS |
| Detachment within the superior 8 clock-hours of the fundus |
Breaks in the inferior >4 clock-hours of the fundus |
| Single break occupying ≤1 clock-hour of the retinal arc |
Single break spanning >1 clock-hour of the retinal arc |
| Multiple smaller breaks within the same quadrant of the fundus |
Multiple breaks spanning >3 clock-hours of the retinal arc |
| Clear media permitting assessment of the retina |
Ocular opacities precluding identification of retinal breaks |
| Absence of advanced glaucoma or clinically significant PVR |
Advanced glaucoma or PVR (grade C or D) |
| Physical and cognitive health, social environment, and lifestyle conducive to prolonged postprocedural positioning |
Disabilities preventing adequate head positioning |
| Aphakia or significant zonular dehiscence |
Surgical Technique
Following is a step-by-step approach to PR. See also Figure 1.
- Carefully examine the eye with indirect ophthalmoscopy and scleral depression to identify areas of pathology.
- Anesthetize areas for cryoretinopexy treatment with subconjunctival anesthesia.
- Perform cryoretinopexy. (Note: Alternatively, retinopexy can be performed using laser photocoagulation once retinal apposition has been achieved after gas injection.)
- Apply povidone-iodine (Betadine) solution to sterilize the injection site.
- Filter perfluoropropane gas (C3F8), sulfur hexafluoride gas (SF6), or air into a tuberculin syringe on a 30-g needle. (See “Selection of Tamponade Agent” for considerations about these gases.)
- Perform an anterior chamber paracentesis to remove 0.1 to 0.25 mL of aqueous humor.
- Select a site perpendicular to the sclera, farthest away from the site of the underlying detachment, and enter 3 to 4 mm from the limbus.
- Withdraw the needle so that only its tip remains in the vitreous cavity, then carefully inject C3F8 (0.2-0.3 mL), SF6 (0.5-0.6 mL), or filtered air (0.8 mL), making sure that the needle tip is not in the suprachoroidal space.
- Reexamine with indirect ophthalmoscopy to confirm placement of gas bubble over the retinal break(s) and perfusion of the central retinal artery (repeat paracentesis if arterial pulsations are absent).
- Review head positioning and gas bubble precautions with the patient, with attention to later expansion of the gas bubble.
Selection of Tamponade Agent
The tamponade agent for PR is selected based on the size and duration of the bubble needed to sufficiently cover all retinal breaks. Sulfur hexafluoride gas (SF6) is commonly used as a tamponade agent in PR. Perfluoropropane gas (C3F8) is more expansible and has a longer duration of action compared to SF6.1,2 Therefore, C3F8 may be preferable for the treatment of larger retinal breaks or multiple smaller breaks.
For small breaks, some surgeons prefer to use filtered air rather than gas, as air produces fewer biochemical and structural changes in the vitreous than does either SF6 or C3F8.1,2 However, because air bubbles do not expand within the vitreous cavity and have a shorter duration of action, the use of filtered air requires a larger-volume injection and, consequently, multiple paracenteses before and after injection to mitigate postinjection elevation in IOP.1
___________________________
1 Sinawat S et al. Arch Ophthalmol. 2010;128(10):1243-1247.
2 Hilton GF et al. Indian J Ophthalmol. 1996;44(3):131-143.
|
Advantages
When results are controlled for anatomic configuration, PR has demonstrated rates of final reattachment comparable to those reported in SB and primary PPV,7,8 although there are no prospective studies directly comparing the three modalities. With judicious case selection, PR offers distinct advantages over SB and PPV.
As an office-based procedure, PR does not use systemic anesthesia or sedation; moreover, it eliminates or reduces the time spent scheduling, waiting for OR and staff availability, and the general discomfort and morbidity associated with surgery. PR also provides a substantial cost benefit, with an estimated cost that is between 25% and 50% of that of PPV and SB (individually or in combination).9,10
For scenarios in which PPV or SB surgery is warranted—such as in cases of RRD complicated by PVR of grade C or D—but access to vitreoretinal surgical facilities is limited, PR may maintain macular attachment until the appropriate surgical team and resources can be allocated.
Disadvantages
In addition to patient cooperation with postprocedural head positioning, successful PR requires a high degree of surgical acumen, aptitude, and experience with indirect ophthalmoscopy and retinopexy. The procedure becomes increasingly difficult when dense cataract, vitreous hemorrhage, or other media opacity obscures the identification of retinal breaks.
The greatest contributor to success in PR is appropriate case selection. Hence, a major limitation to using this safe, low-cost, and well-tolerated office-based procedure is its relative lack of generalizability to all cases of RRD.
Gas bubble displacement. It is not uncommon for the expansile gas bubble to move and displace the vitreous.4 This displacement may create new breaks or reopen a break that was just treated, either of which may lead to failure of reattachment. Another occurrence unique to PR is the formation of smaller gas bubbles (“fish eggs”),9 which have the potential to enter the subretinal space through the existing retinal break(s).4
Pearl. Proper gas injection technique and careful indirect ophthalmoscopy are essential for preventing formation of fish-egg bubbles. The needle should penetrate the eye perpendicular to the sclera, and at least three-quarters of the needle should be withdrawn prior to injection. This makes it easier for the gas emerging from the shaft to enter the vitreous cavity as a single coalesced bubble. If small bubbles do form, light strokes on the sclera with a cotton-tipped applicator may break the surface tension of the bubbles.
Alternatively, the patient can position his or her head so that the fish-egg bubbles are localized away from the break(s). This will allow for spontaneous coalescence of the bubbles, typically within 24 hours. The patient can then resume appropriate positioning for tamponade of the break(s).
Outcomes
Since the inception of PR more than 30 years ago, its overall single-operation success rate (SOSR) has reportedly increased from 55% to between 75% and 80%,7,9 likely due to more stringent patient selection by retina specialists. Nevertheless, the SOSR for PR remains lower than the 83% to 85% rate reported for PPV or combined SB/PPV and the 75% to 91% reported for SB.7,8 This may be attributed in part to unidentified breaks, which are most often the cause of persistent or recurrent RD after PR in appropriately selected cases of uncomplicated RRD.
Given these statistics, a majority of retina specialists often choose to perform SB, PPV, or both instead of PR. However, SOSR should not be the sole criterion in selecting the treatment for uncomplicated RRD. Studies have shown that the rate of final reattachment with surgical intervention or PR in appropriately selected patients is greater than 95%.11,12 Moreover, performing PR as a first-line treatment eliminates an OR visit, reduces cost to the patient and health care system, and offers the potential for rapid improvement in visual acuity.3,13
Reoperation. Even in cases in which an initial failure required reoperation with repeat PR or with SB or combined SB/PPV, patients who had first undergone PR have been reported to achieve better visual outcomes compared with SB alone.9 We believe this is likely attributable to the potential for earlier macular reattachment, as well as purposeful head positioning, which inhibits further accumulation of subretinal fluid.
Furthermore, the use of PR does not affect the patient’s ability to undergo later PPV or SB9,13; thus, those failing primary PR treatment remain viable candidates for reattachment with a surgical procedure. The rates for most postoperative complications with PR—including the development of PVR, cystoid macular edema, diplopia, and epiretinal membrane—are equal to or less than that of SB and PPV.7
Pearl. If retinal apposition has not been achieved after PR and reoperation with PPV, SB, or both is needed, the patient should be advised to avoid supine positioning in the immediate preoperative setting. Prolonged contact of intraocular gas with the posterior lens surface may cause lens feathering with posterior subcapsular changes,14 which can contribute significantly to poor intraoperative visibility.
Conclusion
PR is a safe, low-cost, well-tolerated, office-based procedure that is often underutilized. Successful outcome is primarily dependent on a thorough retinal examination that identifies all breaks and on careful patient selection. With these criteria in mind, physicians may opt for PR as a first-line treatment and nonsurgical alternative to SB, PPV, or combined SB/PPV in patients with uncomplicated superior RRD.
___________________________
1 Hilton GF, Grizzard WS. Ophthalmology. 1986;93(5):626-641.
2 Tornambe PE et al. Ophthalmology. 1988;95(5):597-600.
3 Goldman DR et al. Ophthalmology. 2014;121(1):318-326.
4 Chan CK et al. Surv Ophthalmol. 2008;53(5):443-478.
5 Chang TS et al. Ophthalmology. 2003;110(3):589-594.
6 Kwon OW et al. Dev Ophthalmol. 2016;55:154-162.
7 Barrie T et al. Br J Ophthalmol. 2003;87(6):782-784.
8 Paulus YM et al. Ophthalmic Surg Lasers Imaging Retina. 2017;48(11):887-893.
9 Tornambe PE. Trans Am Ophthalmol Soc. 1997;95:551-578.
10 Chang JS, Smiddy WE. Ophthalmology. 2014;121(4):946-951.
11 Kinori M et al. Am J Ophthalmol. 2011;152(2):291-297.e2.
12 Ross WH, Lavina A. Can J Ophthalmol. 2008;43(1):65-72.
13 Tornambe PE et al. Ophthalmology. 1989;96(6):772-784.
14 Mohamed S, Lai TYY. Hong Kong J Ophthalmol. 2010;14(1):8-13.
___________________________
Dr. Moinuddin is a preliminary medicine resident/future ophthalmology resident at the University of Michigan W.K. Kellogg Eye Center. Dr. Wubben and Dr. Besirli are assistant professors, and Dr. Zacks is a professor in the Department of Ophthalmology and Visual Sciences at the University of Michigan W.K. Kellogg Eye Center.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Besirli iRenix Medical: C,O,P; ONL Therapeutics: P.
Dr. Moinuddin None.
Dr. Wubben None.
Dr. Zacks ONL Therapeutics: C,O,P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|