Download PDF version
The advent of anti-VEGF therapy has revolutionized the treatment of patients with neovascular age-related macular degeneration AMD, but it has also led to a range of therapeutic approaches among retina specialists, with limited consensus on best practices. This is true even for patients who have a robust response to one of the main anti-VEGF drugs, ranibizumab Lucentis or bevacizumab Avastin.1 So when it comes to patients who don’t respond—or have lost responsiveness—the absence of evidence-based guidelines makes treatment decisions all the more challenging.
“It’s not like there are hard-and-fast rules,” said Susan B. Bressler, MD, professor of ophthalmology at Wilmer Eye Institute, Johns Hopkins University. “Every patient is different, and every doctor is shooting from the hip right now when treating refractory patients.”
Apart from treating these patients, even just defining “nonresponders” varies among clinicians, which adds to the complexity of this issue, said Amani A. Fawzi, MD, associate professor of ophthalmology at Northwestern University in Chicago. Among the complicating factors are the unknown causes of nonresponsiveness, as well as financial and treatment burdens.
Fortunately, anti-VEGF drugs work well for the vast majority of neovascular AMD patients. It is only a minority of patients in whom loss of reactivity is a problem.2
Complicating Factors
Terminology confusion. There is no universally accepted nomenclature for describing different types of nonresponsiveness. “This is the very first problem with delving into this topic. It’s difficult to compare outcomes when we’re defining things differently from one another, let alone figure out what we can do to improve outcomes,” said Dr. Bressler.
The terms tachyphylaxis and tolerance are both used to describe a decreasing therapeutic response to a pharmacologic agent. Some authors use the words synonymously, while others make distinctions based on the mechanism and time course—with tachyphylaxis denoting rapid onset over a short period and tolerance developing more slowly.3 But there are also patients who don’t respond from the start true nonresponders and people who take a drug holiday after successful treatment but cease to respond when re-treated.
“At the end of the day, the important thing is that there is a group of people who are not responding well to the drug, albeit a small group. Whether it’s tolerance or tachyphylaxis or something else, what we care about is finding something they do respond to,” said Sander R. Dubovy, MD, associate professor of ophthalmology and pathology at the Bascom Palmer Eye Institute.
Financial costs and treatment burden. In clinical practice, few retina specialists adhere to the strict schedule of regular monthly intravitreal anti-VEGF injections for two years, as established by the two major trials of ranibizumab. Variable regimens have become the de facto practice because of the financial costs of the drug and procedure, patient preferences, and practice workload.1
“Discussing anti-VEGF drugs without mentioning their financial burden is like ignoring the elephant in the room,” said Dr. Bressler. “It would be naive to think that the financial burden and practice burden of anti-VEGF agents don’t influence drug choices and treatment schedules.” These same issues affect how clinicians treat refractory patients.
The average drug cost per injection is about $50 for Avastin, $2,000 for Lucentis, and $1,850 for the recently approved Eyelea Regeneron. Overall treatment costs will vary depending on the dosing regimen and possible manufacturers’ reimbursement programs.
Unknown factors in nonresponsiveness. “Until we know what’s actually happening to cause a lack or loss of efficacy, it’s difficult to determine the best way to counter the problem,” said Dr. Dubovy. “It may be that there are structural differences or changes in the retina that lead to differences in response, such as increased fibrosis that acts as a barrier to fluid resorption.”
“Our therapies may be blocking the VEGF pathway to the point that a parallel angiogenic mechanism is upregulated in the membrane, enabling continued growth despite anti-VEGF therapy,” said Dr. Fawzi. “This is the whole premise behind doing combination therapy and studying new drugs with different mechanisms of action.”
|
Switching Treatments
|
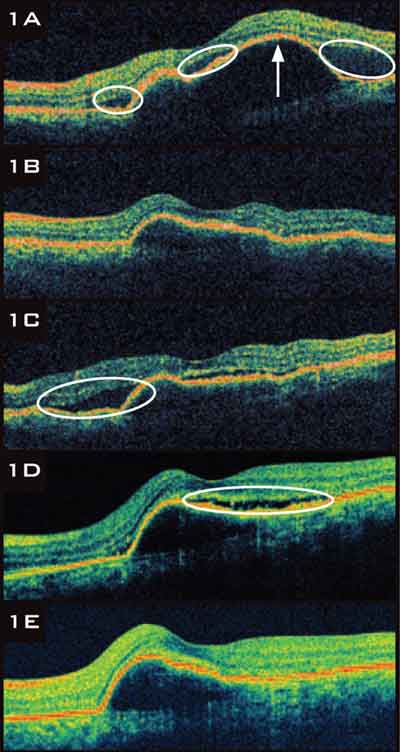 |
RANIBIZUMAB TACHYPHYLAXIS.
1A A 65-year-old with neovascular AMD presented with subretinal fluid SRF, circled and a large serous pigment epithelial detachment PED, arrow. 1B After three ranibizumab injections, SRF resolved completely, and PED size decreased. 1C SRF recurred. 1D Despite six more ranibizumab injections, SRF and PED persisted. 1E The patient was switched to bevacizumab. After six injections, SRF resolved, but the PED remained. |
Spectrum of Approaches
Clinicians currently have several options for managing a poor response to anti-VEGF injections. These include reducing treatment intervals, giving the patient a drug holiday, combining therapies with different modes of action, or switching to a different drug.3
In light of a recent study analyzing the outcome of switching anti-VEGF drugs, the last option is currently at the forefront of discussion. Researchers reported that, among patients who were treated primarily with either ranibizumab or bevacizumab and who showed an attenuated response, switching to the other drug was successful in continuing to reduce fluid in 81 percent of cases.4 These findings are surprising given that ranibizumab and bevacizumab are similar molecules that act at the same location.3 However, this promising news is tempered by some limitations noted in the study, including retrospective design and relatively small patient population.
Each of the three AMD experts interviewed for this article takes a different therapeutic approach to the problem of nonresponsiveness.
Dr. Fawzi switches anti-VEGF drugs; may add PDT. Dr. Fawzi, co-author of the study mentioned above, treats her refractory patients according to the study protocol. She said:
“We treat our neovascular AMD patients until they are completely dry; we don’t tolerate any fluid in the subretinal space. When patients are dry, we take a drug holiday but continue to follow them on the same schedule. If fluid returns or vision drops, we resume treatment with the same drug that worked before. If a patient doesn’t respond to the drug when it’s resumed, then we consider him or her a nonresponder these patients were not included in our study.
“Patients on anti-VEGF therapy who improve initially and are on their way to becoming dry but then start accumulating fluid again are considered to have tachyphylaxis these patients were included in our study. We might give another couple of injections of the same drug to convince ourselves that what’s really going on is tachyphylaxis, and if the loss of responsiveness continues, at that point we switch to the other anti-VEGF agent. In our study, we saw that 50 percent of patients got better with the first injection just by switching from ranibizumab to bevacizumab or vice versa.
“For the subset of patients with polypoidal lesions, the Asian literature suggests that photodynamic therapy PDT is superior to anti-VEGF therapy. We have found that this group responds much better to either a combination of PDT and anti-VEGF closing the polyps with PDT helps the anti-VEGF effect or full-dose PDT alone. My approach is to use half-dose PDT every three months in combination with anti-VEGF on its standard schedule.”
Dr. Dubovy considers different schedules, alternating drugs. Dr. Dubovy’s approach focuses on the dosing schedule. This is not surprising given that he was coauthor of the PRONTO study,5 which had a strong influence on the widespread adoption of alternative variable-dosing regimens. Dr. Dubovy said:
“If patients are not responding, a reasonable thing to do is to bring them back in a week or two rather than a month to assess whether they’re dry at that interval. If they are, then you know they have responded to the drug and perhaps need more frequent injections. If fluid is present in that short interval, then you know they are true nonresponders.
“For patients with attenuated response, some have advocated more frequent dosing. This often solves the problem. In some cases, alternate dosing between ranibizumab and bevacizumab every two weeks has anecdotally been successful. When dry, the patients are returned to a four-week schedule on the original drug they responded to. Once back on a monthly schedule, some patients revert, but some don’t. An every-two-week dosing schedule deviates significantly from the standard schedule, so some clinicians are uneasy about it.
“Switching back and forth between ranibizumab and bevacizumab has not been a major concern because the drugs are very similar. If you look at the data, Lucentis probably dries the retina a little bit better so that patients need slightly fewer injections, but essentially they work about the same.”
Dr. Bressler sticks with ranibizumab. Dr. Bressler treats her patients almost exclusively with ranibizumab and doesn’t necessarily consider residual fluid a reason to make a change. She said:
“I’m a ranibizumab-first person, unless there’s a financial barrier from the patient’s perspective, but most of my patients have secondary insurance. I see no rationale in switching from ranibizumab to bevacizumab when the CATT study shows no suggestion that bevacizumab is superior to ranibizumab in terms of vision; and, anatomically, it appears it may be inferior to ranibizumab.6
“In what I consider to be refractory cases—those in which the vision, angiogram, and OCT are essentially unchanged after about nine to 12 consecutive monthly injections—the first thing I do is check whether the patient has been coming in religiously within the three- to five-week window established as the treatment interval in phase 3 studies of ranibizumab. If the drug hasn’t been administered consistently within that window, then it hasn’t been used in the fashion in which it was demonstrated to work. If the schedule is off, I correct it. If it’s fine, then I might hedge the treatment interval closer to the three-week mark for an additional consecutive series of injections.
“Does it distress me that some patients have fluid after 12 consecutive injections? Sure. But it would be pretty hard to argue that I should jump ship if, over the course of that 12 months, their vision had improved. Maybe they’re not 20/20, but they’ve gained a couple of lines of acuity, they have far less fluid at month 12 than when they started, and they show no leakage on their angiogram.
“By contrast, in cases where I’ve given consecutive monthly injections of reasonably long duration, but it looks as if I’ve been doing absolutely nothing other than maintaining the status quo, I’m more apt to ask myself what to do next. After confirming that treatments have been administered at three- to five-week intervals, in the past I have considered adding PDT to continued ranibizumab therapy. Although I was more excited about this particular combination therapy when I had a few successes, the more I’ve tried it, the less enthusiastic I’ve become. Controlled trials have not shown that combination PDT plus ranibizumab provides advantages when compared to ranibizumab monotherapy.”
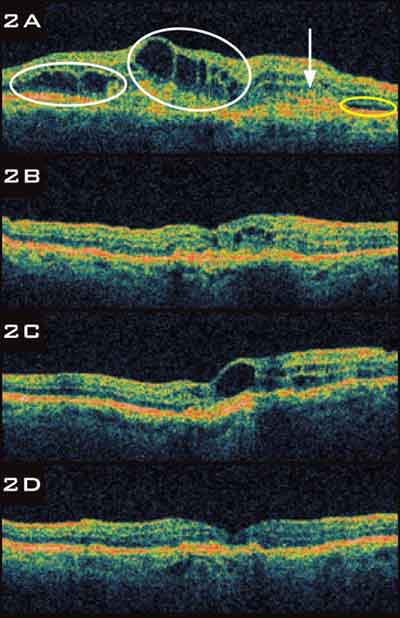 |
BEVACIZUMAB TACHYPHYLAXIS.
2A An 85-year-old with neovascular AMD presented with cystic retinal edema circled in white, SRF circled in yellow, and a fibrovascular PED arrow. 2B After two treatments with bevacizumab, retinal edema improved and SRF resolved. 2C Despite three more treatments with bevacizumab, cystic retinal edema worsened. 2D The patient was switched to ranibizumab, and cystic retinal edema resolved after three injections. |
How Eylea Fits Into the Picture
Eylea, formerly known as VEGF Trap-Eye aflibercept, is a protein that acts as a decoy receptor for VEGF. The recommended dosing is once every four weeks for the first three injections, followed by once every eight weeks thereafter. This reduced frequency of injections is considered by many to provide a clear advantage.
The FDA approved Eylea in November 2011. Although it is not yet widely adopted, Dr. Dubovy has switched over some of his patients. “Reimbursement is currently only approved for patients who’ve been on Lucentis and have residual fluid. So I’ve started with that subset, and the group appears to be doing very well.”
Most experts agree that Eylea will not be the go-to drug for treatment-naive patients, largely because of reimbursement issues. The first patients for whom the drug will be recommended are most likely to be those with inadequate response to other anti-VEGF therapy. It remains to be seen how Eylea will behave in such patients. Compared with the population that participated in the phase 3 trials assessing Eylea, refractory patients may be different genetically or may have a highly mature membrane that does not respond to anti-VEGF drugs, said Dr. Fawzi. Because her study found that 50 percent of patients with tachyphylaxis got better with the first injection after switching between ranibizumab and bevacizumab, if she doesn’t see a benefit after the first post-switch injection, she plans to move to Eylea right away.
Dr. Bressler also plans to incorporate Eylea in her clinical practice by switching over her more refractory patients. “As I gain this experience, I’ll initiate therapy in some treatment-naive patients, assuming there are no financial barriers.”
Drs. Dubovy and Fawzi cautioned that, eventually, cases of attenuated response to Eylea will probably emerge, so therapies with different modes of action are still very much needed.
___________________________
Dr. Bressler reports that Johns Hopkins University School of Medicine receives research grants from Genentech, the manufacturer of Lucentis and Avastin. Drs. Dubovy and Fawzi report no related financial interests.
___________________________
1 Bhisitkul RB, Stewart JM. Expert Rev Ophthalmol. 2010;56:799-809.
2 Eghøi MS, Sørensen TL. Br J Ophthalmol. 2012;961:21-23.
3 Binder S. Br J Ophthalmol. 2012;961:1-2.
4 Gasperini JL et al. Br J Ophthalmol. 2011;961:14-20.
5 Fung AE et al. Am J Ophthalmol. 2007;1434:566-583.
6 Martin DF et al; CATT Research Group. N Engl J Med. 2011;36420:1897-1908.