This article is from September 2012 and may contain outdated material.
“No pain, no gain,” as the saying goes. But coping with the “pain” of refractive surgery crises is no easy feat, even for experts in the field. The good news is that many catastrophes can be managed successfully—or even avoided—if proper measures are taken. And, of course, learning the hard way leads to greater knowledge and better patient outcomes in the future. Here, several experts recount difficult situations, how they handled them, and what they’ve learned from their experience.
Case 1: A Drastic “Cure” for Poor Follow-up
Richard J. Duffey, MD, handles a case of overseas LASIK gone wrong.
PATIENT DETAILS Nine months after having undergone bilateral LASIK overseas, at the end of military deployment, a 33-year-old man sought help for loss of uncorrected visual acuity (UCVA) in his left (nondominant) eye. The acuity fluctuated widely and was recorded as 20/80. Best-corrected visual acuity (BCVA) in his left eye was 20/50. The surgeon who performed the LASIK procedure had used a mechanical microkeratome, but there was no documentation of the make or model. The patient reportedly had no follow-up since undergoing the refractive surgery.
WHAT WENT WRONG? The right eye, which had been treated first, showed a healed corneal flap scar and had stable UCVA of 20/20. However, the nasal-hinged flap of the left eye was very thin, due to necrosis after extensive epithelial ingrowth. The flap had 360 degrees of ingrowth and a very irregular edge, with five to six clock hours of edge melt. Only the very center of the flap was free of epithelial growth.
Surgical records were not available, but I suspected that the original flap was irregular and too thin, perhaps because of inadequate microkeratome suction.
ADDRESSING THE PROBLEM I doubted the usefulness of conservative treatment (lifting the flap to remove the necrotic tissue, then replacing the flap) because the residual flap was extremely thin. The only viable approach appeared to be a drastic one: amputating the flap. Phototherapeutic keratectomy (PTK) would then smooth the residual stroma.
After thorough consultation with the patient, and with his consent, I amputated the thinned necrotic flap of his left eye. I moistened the bare stroma with a diluted methylcellulose artificial tear and performed PTK (ablation depth of 10 µm) over a 6-mm optical zone, with a transition zone of 0.5 mm. Before inserting the bandage contact lens, I applied mitomycin C (0.02 percent) for two minutes, to retard scarring.
OUTCOME The epithelium healed by 10 days postoperatively, with no evidence of irregularities. The patient’s UCVA at one month was 20/40. At the three-month and one-year follow-up exams, it was 20/25. The patient’s only complaint was that the vision in his left eye was not quite as good as in the right eye.
LESSONS LEARNED This was the only case in which I’ve had to amputate a LASIK flap. Many surgeons would not consider doing so. But I believe that amputation can be a good option when extensive epithelial ingrowth has occurred.
I was relieved that the procedure worked as well as it did, and some of the credit is due to the specific circumstances of this case. Fortunately, almost all the stromal necrosis was in the flap rather than the bed. So the stromal bed was fairly smooth to begin with, and PTK made it even smoother.
Of course, the better scenario would have been for the ingrowth to be detected about one month postoperatively, by which time this complication usually is apparent. But even today, in the United States, some discount LASIK centers effectively discourage patients from seeking follow-up by charging them extra for LASIK postoperative exams.
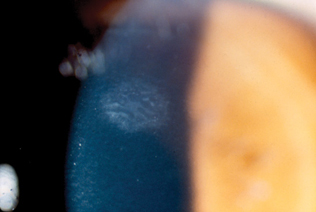 |
|
Epithelial Ingrowth. If epithelial ingrowth is identified early, as in the case shown here, the more extensive ingrowth and visual consequences encountered by Dr. Duffey can be avoided.
|
Other Views
RICHARD L. LINDSTROM, MD Epithelial ingrowth is very rare in primary LASIK cases. But if flap-lift LASIK enhancement is performed more than two years after the initial procedure, epithelial ingrowth occurs in up to 8 percent of eyes, and in many cases it’s aggressive.
Epithelial cells have tight junctions, so a sheet of ingrowth can obstruct the flow of nutrient-rich aqueous to the stroma. That obstruction, along with collagenase release, is what leads to necrosis of the stromal cells and corneal flap.
If there is a continuous sheath of epithelium under the flap, with associated corneal melting, then scraping out the epithelium often won’t work. If this is done, severe irregular astigmatism usually occurs. I’ve had four or five cases in which I had to simply amputate the flap, smooth the bed with a little PTK, and then let the cornea heal.
Afterward, compensatory epithelial hyperplasia smooths the surface in the short term; and, over two to three years, the cornea itself also can undergo some slow remodeling. I usually find that the patient’s vision slowly and continuously improves for up to five years, and excellent visual outcomes can be achieved. In addition, once a stable refractive outcome is attained, an enhancement with photorefractive keratectomy (PRK) can be done with adjunctive mitomycin C.
It’s interesting that the cornea has this ability for healing and remodeling. It’s amazing that these cases can turn out pretty well.
AMAR AGARWAL, MBBS, MS, FRCS, FRCOPHTH (LON.) Dr. Duffey’s approach is correct. If the surgeon attempts to preserve a necrotic flap, the cornea will not heal. But if the epithelial ingrowth is in the early stages, it is possible to simply lift the flap, clean out the epithelial cells, and reposition the flap.
Preventing recurrence. Some surgeons seal the tract around the flap with fibrin glue to prevent recurrent ingrowth. Another technique (which I have not tried) is being used by Jorge Alió, MD, and colleagues in Alicante, Spain.1 Dr. Alió has reportedly reduced the recurrence rate by irradiating hyperplastic epithelial cells with an Nd:YAG laser.
The bottom line is that we should not allow epithelial ingrowth to advance as far as it did in this patient.
___________________________
1 Avala MJ et al. Am J Ophthalmol. 2008;145(4):630-634.
Case 2: Mistakes Happen, So Use Checklists
Sonia H. Yoo, MD, corrects a human error that could have been avoided.
PATIENT DETAILS A 50-year-old woman, upset over poor visual acuity in one eye after bilateral LASIK elsewhere, presented six weeks postoperatively for re-treatment. She had lost confidence in the original surgeon after learning that a mistake in data entry had occurred preoperatively. UCVA in her problem eye was 20/40 –2; BCVA was 20/25.
WHAT WENT WRONG? In her initial surgery, the excimer laser had been set to treat the wrong axis of astigmatism. The axis refractive treatment of her right eye was input into the laser in minus cylinder instead of plus cylinder notation. The original manifest refraction was –4.25 + 1.25 X 165. Treatment entered into the excimer laser was –4.00
–1.25 X 165. Postoperatively, manifest refraction was plano + 2.00 X 166.
ADDRESSING THE PROBLEM The patient’s previous surgical experience had made her very anxious, so I treated her two months later, which is a bit earlier than I usually do.
The treatment corrected +2 D of astigmatism at 166 degrees.
OUTCOME After re-treatment, the patient’s UCVA was 20/25 and BCVA was 20/20 –2.
LESSONS LEARNED This case demonstrates the importance of instituting and following operating room protocols to guard against avoidable mistakes in refractive surgery. Although the equipment we use is high tech, manual data entry can introduce human error and adversely affect outcomes.
Use a checklist. Like airline pilots, ophthalmic surgeons can guard against disaster by creating and consistently using a checklist. An essential item to be included on this list: verifying, in the procedure room, the accuracy of all machine settings and of the planned treatment. These checks must be done separately for each eye, of each patient, every time, and in the same manner. Although distractions may interfere, it’s very important to stay disciplined and focused.
Cross-check each other. In my surgical practice, we check and double-check settings and treatment plans just before the refractive surgery begins. First I go through the safety checklist; then the technician (my “copilot”) goes through the same checklist, independently, to double-check me. So if I miss something, hopefully my copilot will catch it.
Other Views
LOUIS D. NICHAMIN, MD I agree with Dr. Yoo completely about following a routine to prevent mistakes. Treating the wrong astigmatic axis is a mistake that is easy to make, and you must be methodical and systematic to avoid it. In more than 20 years of performing astigmatic refractive surgery, I have once programmed the laser incorrectly and once placed limbal relaxing incisions on the wrong meridian. When such an error occurs, it is almost always on the opposite axis, leading to a doubling of the patient’s preoperative astigmatism.
The most common error I’ve seen in astigmatism-correcting surgery is putting the correction in the wrong axis. Likewise, from teaching residents to perform limbal relaxing incisions after cataract surgery, I’ve found that when a resident makes a mistake, it is almost always that the astigmatic correction is 90 degrees from where it should be.
When this type of mistake occurs, the surgeon should be completely honest with the patient about what happened. It is appropriate to apologize to the patient and explain that human error was the cause. You should not be reticent. Let the patient know that you care and that you will help rectify the situation.
Put up a picture. A topographic map provides a visual landscape of the astigmatism. Having topography on display in the operating room is helpful. I urge beginning surgeons to place a topographic image of the eye directly in front of them, in the same orientation as their view of the patient’s eye. I’ve had colleagues share that this has saved them more than once.
AMAR AGARWAL, MBBS, MS, FRCS, FRCOPHTH (LON.) I would treat this patient’s astigmatism as though it were her first refractive surgery in that eye; whatever the existing refraction is, that is what I would treat.
Doctors must take responsibility. As Dr. Yoo mentioned, it is not uncommon for human error to be introduced when data are entered into a machine. This type of mistake can happen if the doctor has an assistant set the laser, then just walks in and performs the surgery without checking the settings. The surgeon must have systems in place to prevent such errors.
To me, the take-home message is this: Enter the data yourself. Don’t depend on your assistant, nurse, or technician to do this. Ultimately, the patient is your responsibility.
Case 3: A Flap Goes to Pieces
A troublesome second LASIK flap is managed by Amar Agarwal, MBBS, MS, FRCS, FRCOphth (Lon.).
PATIENT DETAILS Three years earlier, this 35-year-old man underwent bilateral LASIK elsewhere, for –5 D to –6 D of refractive error. Records from the procedure were not available. The patient desired secondary LASIK to eliminate residual error of –2 D with –1 D cylinder.
WHAT WENT WRONG? Instead of lifting the existing flap, I decided to make a femtosecond laser flap at a depth of 120 µm in each eye. I had performed anterior segment OCT and had calculated the flap from the previous surgery to be 150 µm. My assumption was that if I made a 120-µm femtosecond flap, I could create a new flap and be safe. How wrong I was!
The femtosecond laser portion of the procedure was uneventful, but the flap lift was more difficult than usual. I knew for certain that I was in trouble when I saw that the flap was coming up in pieces. It was separating irregularly along both planes, at 120 µm and 150 µm. I realized that I had gotten myself into a nightmare situation.
ADDRESSING THE PROBLEM My only choice was to excise a small area of the fragmented flap manually, perform the excimer laser ablation, and hope for the best.
I also had to operate on the patient’s other eye. I knew that if I created a 120-µm flap for that eye, the same problem could occur. So I programmed my laser for a 100-µm flap, more anterior than the previous flap. Luckily, the surgery on that eye went smoothly. But I could not sleep that night because of my experience with the first eye.
OUTCOME At the patient’s postoperative exam the next day, his visual acuity was 20/20 without glasses, in both eyes. His vision has been stable for three years.
LESSONS LEARNED It was a mistake to try to create a new femtosecond flap on an eye that already had undergone LASIK. I should have performed photorefractive keratectomy (PRK).
The moral of the story: If your patient has already undergone LASIK, you should either lift the existing flap or perform surface ablation. Don’t make the mistake I did. You might get away with it, but what if you don’t?
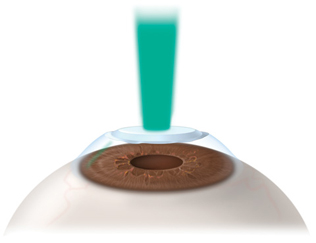 |
|
Lesson Learned. Instead of creating a second flap, Dr. Agarwal advises using PRK to correct residual refractive error after LASIK.
|
Another View
LOUIS D. NICHAMIN, MD Creating a new flap on a cornea that already has had LASIK is something that most refractive surgeons have learned the hard way not to do. Nor is it typically advisable to lift the existing LASIK flap for the second refractive procedure because this is associated with epithelial ingrowth. Consequently, most refractive surgeons would consider PRK to be the treatment of choice in this situation.
Dr. Agarwal’s logic was sound in scanning the cornea to estimate the original flap’s depth and adjust the femtosecond cut accordingly, but the result demonstrates that the inherent variables in such a case are high. What is also interesting is that Dr. Agarwal had the courage to proceed with surgery on the patient’s other eye, albeit at a different depth.
Although everything ultimately turned out well, I would have been inclined to call it a day after the first flap. I’ve found that discussing the findings and reviewing additional options with the patient, without the effects of sedation, have led to the best outcomes in such complex cases.
Case 4: An Intacs Segment Travels
Anterior migration of an intracorneal ring segment is addressed by Soosan Jacob, MS, FRCS, DNB.
PATIENT DETAILS Femtosecond-facilitated implantation of asymmetric intracorneal ring segments (ICRS) of 0.21 mm thickness superiorly and 0.40 mm inferiorly (Intacs, Addition Technology) was planned in a 25-year-old keratoconus patient whose preoperative UCVA was 20/100 and BCVA was 20/30.
Insertion of the superior 0.21-mm segment was less than satisfactory. Unusual resistance made it difficult to insert the segment, and excessive force was required. However, the procedure eventually ended with both Intacs segments in the correct position. Postoperatively, UCVA improved to 20/30 and BCVA to 20/25.
WHAT WENT WRONG? Seven weeks postoperatively, I observed that the superior segment had migrated anteriorly and was overlapping the other segment slightly. This progressively increased over the next four months and was confirmed by anterior segment optical coherence tomography (AS-OCT). Six months postoperatively, melting was visible in the overlying cornea.
ADDRESSING THE PROBLEM To prevent further corneal damage, the ring segments were removed and the cornea was allowed to heal.
OUTCOME The patient had a superficial scar and thinning in the area of melt, which was outside the visual axis at the 7-mm zone. BCVA was maintained at 20/25 through the last follow-up exam two years after the surgery.
LESSONS LEARNED The femtosecond laser has enabled cornea surgeons to place ring segments more accurately in the cornea, inside a 360-degree, precisely horizontal stromal channel. But if the surgeon maneuvers a segment inappropriately during insertion, the tip can begin cutting an angled, false channel.
Angulation may lead to perforation. Typically, the false channel prevents the device from moving forward, leading to suboptimal placement of the ICRS. The angulation of a false channel also orients the segment tips toward the corneal surface or the anterior chamber, with associated risks for perforation if the segment begins migrating.
After experiencing false channels in two of my ICRS patients, I developed an intraoperative solution to false channelization that I call the turnaround technique.1
Warning signs and solutions. If inserting the first segment becomes difficult, or if radiating stromal folds and striae appear around its advancing tip, this indicates a false channel. In such cases, I immediately remove the segment, then turn it around and insert it in the opposite direction through the entry incision. Because the rerouted segment must travel nearly 360 degrees along the channel, the second segment is used as an intrachannel pushing instrument.
For cases in which asymmetric segments are planned and the false channel is created with the second segment, a double-pass turnaround technique is performed. Here, the second segment is used to push the first one forward. The first one is then pulled out through the incision and is reintroduced to push the second one into its final intended position. An alternative is to pull the first one out, then perform a turnaround technique with the second segment.
___________________________
1 Jacob S et al. J Cataract Refract Surg. 2010;36(8):1253-1260.
Another View
WILLIAM B. TRATTLER, MD When a femtosecond laser is used to create the channels for Intacs, the channel extends 360 degrees. Typically, the height and width of the channel are optimized for the size of each Intacs segment so that the Intacs can be carefully inserted and snugly fitted. This provides the optimal method for ending up with a positive change in the abnormal corneal topography shape.
However, as Dr. Jacob noted, an insertion that is more difficult than normal could indicate that the ICRS is cutting a false channel. She describes a technique that makes a lot of sense if the ring segments appear to be straying outside the intended channel in the stroma. This method could be effective for ensuring proper placement and for averting complications from a segment migrating to the surface or into the anterior chamber.
Fortunately, I haven’t experienced false channelization during an Intacs insertion procedure, but I’m glad to know that there’s a way to manage it. Knowledge of this method would certainly come in handy if Intacs insertion does not proceed as expected, and I appreciate Dr. Jacob sharing her thoughts on this remedy.
Case 5: A Catastrophic Refractive Lens Exchange
Luis Izquierdo Jr., MD, PhD, deals with a case of expulsive hemorrhage.
PATIENT DETAILS In 2002, a 38-year-old man sought a surgical solution to high hyperopia (+15 D right eye, +16 D left eye), with emmetropia as the goal. Preoperatively, his UCVA was 20/400; BCVA was 20/40 in the right eye and 20/50 in the left. Axial lengths were 15.93 mm in the right eye and 15.92 mm in the left. The patient also had mild bilateral nystagmus.
I recommended refractive lens exchange (RLE), first on the left eye, with piggybacking of a +24 D silicone intraocular lens (IOL) and a +30 D acrylic IOL (the only option available at the time).
WHAT WENT WRONG? The surgery was routine, and piggyback IOLs were implanted successfully. The patient’s UCVA was 20/40 at his three-day postoperative exam. However, he returned at one week after surgery, complaining of blurred vision. Examination showed intraocular inflammation, with residual cortex in the anterior chamber. I decided to aspirate the residual cortical material with a bimanual irrigation/aspiration technique. As soon as I inserted the handpiece through a side port, the anterior chamber shallowed abruptly, his intraocular pressure (IOP) spiked, and the globe hardened, indicating suprachoroidal hemorrhage, which mandated immediate suturing to prevent expulsion of the eye’s contents.
ADDRESSING THE PROBLEM I quickly withdrew the handpiece and began suturing the incision. However, before the suturing was complete, blood and intraocular contents (including the retina) were expelled rapidly. The suprachoroidal hemorrhage had converted to an expulsive choroidal hemorrhage.
OUTCOME Although the patient has complete loss of vision in the operated eye, enucleation was not necessary. RLE for his other eye was canceled.
EPILOGUE The patient returned one month later with vision loss in the surviving eye. BCVA had declined from 20/50 to 20/200. Posterior OCT showed a serous macular detachment. Steroids were replaced with anti-anxiety medication, and BCVA began improving within days. It returned to 20/50 over the subsequent two months.
LESSONS LEARNED Prior to elective refractive surgery, BCVA was 20/40 in the operated eye, but afterward the eye was blind. This disaster has made me more cautious about performing RLE surgery. I advise patients to wait until they’ve developed an early cataract to have their natural lens replaced with an IOL.
At minimum, surgeons should screen RLE candidates for possible risk factors for choroidal hemorrhage as described by Kuhn et al.1 My patient’s risk factors included very short eyes, excessive weight, and systemic hypertension. He also had a type A personality, which I suspect is another risk factor because of the relationship between anxiety (or stress) and arterial hypertension. For such patients, intraoperative sedation is mandatory in my practice.
In at-risk patients, I use intraocular mannitol, a diuretic, to decrease intraoperative IOP, and I stay alert for early signs of trouble, including anterior bulging of the vitreous and an increase in blood pressure.
___________________________
1 Kuhn F et al. Ophthalmol Clin North Am. 2001;14(4):639-650.
Rx for SCH
In their 2001 review article,1 Dr. Kuhn and colleagues provided these tips for managing intraoperative suprachoroidal hemorrhage (SCH):
- Accept that SCH can happen to you (not just to other surgeons).
- Be aware of the risk factors for SCH.
- Know the early signs of SCH and look for them.
- Design a management plan in advance. (Their suggested first step is to apply pressure to the wound lips, using fingers or strong forceps.)
- Have the necessary tools and materials readily available at all times during surgery, including 8.0 suture material and a Cobo temporary keratoprosthesis or Byrne lens (Ocular Instruments). (The latter allows suturing while still in place.)
- Be prepared to implement the plan.
- If SCH occurs, follow the management plan immediately.
___________________________
1 Kuhn F et al. Ophthalmol Clin North Am. 2001;14(4):639-650.
|
Other Views
MICHAEL LAWLESS, MBBS, FRANZCO, FRACS Dr. Izquierdo is to be commended for presenting this devastating case of expulsive hemorrhage, which involved secondary intervention just one week after the original surgery.
When expulsive hemorrhage occurs in the operating room, it might be somewhat beneficial to attempt decompression via the transscleral route. However, in Dr. Izquierdo’s case, the speed of the expulsive hemorrhage was so fast that this might not have helped.
The patient was not an ideal candidate. A distinction should be made between RLE for hyperopia and this particular case. Axial length was less than 16 mm in each of the patient’s eyes, so his eyes were essentially nanophthalmic. It is very difficult to operate on these eyes, which have shallow chambers and thus are at risk for choroidal effusion postoperatively, which can persist for a long period. It is possible that choroidal leakage and/or effusion were present at the time of secondary intervention.
The key message: Although RLE for hyperopia is a very safe and accurate procedure to correct a refractive error, special consideration must be given to its associated risks when applied to eyes that are structurally abnormal.
WILLIAM B. TRATTLER, MD Not all intraoperative suprachoroidal hemorrhages will result in visual catastrophes. For example, in the case of a 77-year-old male cataract patient with suprachoroidal hemorrhage, I successfully closed the wound and prevented expulsion of the intraocular contents. The man’s vision improved from counting fingers acuity shortly after surgery to BCVA of 20/20 by four months postoperatively.
Case details. My patient underwent phacoemulsification and placement of toric IOLs to correct +2 D of astigmatism. Both procedures were uneventful. I had removed the viscoelastic; and, as I was trying to rotate the IOL into correct alignment, the chamber suddenly shallowed, the red reflex disappeared, the eye hardened from an apparent extreme increase in IOP, and the patient reported sudden significant eye pain. Because I had already experienced a similar case, I was aware that these were signs of suprachoroidal hemorrhage. Therefore, I immediately stopped the rotation maneuver and closed the wound as quickly as possible with two 10-0 nylon sutures. Later that day, the patient’s IOP measured 52 mmHg. My retina specialist advised that high IOP would help prevent further expansion of the hemorrhage. Five days postoperatively, UCVA in that eye was 20/200; it declined to counting fingers by two weeks.
However, the patient’s IOP and acuity improved slowly over the subsequent four months, as the hematoma behind his retina resolved. The four-month exam showed BCVA of 20/20, with residual astigmatism of 0.75 D.
The initial dramatic loss of vision had been of great concern to the patient. But, fortunately, the IOL was close to the intended axis and the hemorrhage did not extend into the macula. Good BCVA was recovered, and both he and I were happy.
Meet the Experts
AMAR AGARWAL, MBBS, MS, FRCS, FRDOPHTH (LON.) Chairman of Dr. Agarwal’s Eye Hospital, Chennai, India, and president of the International Society for Refractive Surgery. Disclosure: Consults for AMO, Bausch + Lomb, and Staar Surgical.
RICHARD J. DUFFEY, MD Cornea and refractive surgeon at Premier Medical Eye Group, Mobile, Ala. Disclosure: Consults for TLC Laser Eye Centers (as an unpaid medical adviser).
LUIS IZQUIERDO JR., MD, PHD Medical director of Oftalmo Salud, Lima, Peru.Disclosure: Receives research funding from NeoVista and NuLens.
SOOSAN JACOB, MS, FRCS, DNB Senior consultant ophthalmologist at Dr. Agarwal’s Eye Hospital and Eye Research Centre, Chennai, India. Disclosure: None.
MICHAEL LAWLESS, MBBS, FRANZCO, FRACS Cornea and refractive surgeon at Vision Eye Institute, Chatswood, Australia. Disclosure: Consults for LenSx (Alcon).
RICHARD L. LINDSTROM, MD Founder and attending surgeon of Minnesota Eye Consultants and adjunct professor emeritus of ophthalmology, University of Minnesota, Minneapolis. Disclosure: Consults for Alcon, AMO, Bausch + Lomb, and TLC Vision.
LOUIS D. NICHAMIN, MD Medical director of the Laurel Eye Clinic, Brookville, Pa.Disclosure: Consults for 3D Vision Systems, Allergan, AMO, Bausch + Lomb, Eyeonics, Foresight Biotherapeutics, Glaukos, iScience Surgical, LensAR, PowerVision, RevitalVision, SensoMotoric Instruments, and WaveTec. Also is an equity owner of 3D Vision Systems, Eyeonics, iScience Surgical, LensAR, PowerVision, RevitalVision, and WaveTec.
WILLIAM B. TRATTLER, MD Cornea specialist with the Center for Excellence in Eye Care, Miami. Disclosure: Consults for Alcon, Allergan, AMO, CXLUSA, Ferrara Ophthalmics, Ista, LensAR, Lenstec, Merck, and Oculus and receives research funding from Bausch + Lomb and Hoya.
SONIA H. YOO, MD Professor of ophthalmology at the Bascom Palmer Eye Institute.Disclosure: Consults for Alcon, Bausch + Lomb, Carl Zeiss Meditec, Croma, and Transcend Medical and receives research funding from Allergan and Genentech.
|