JAN 12, 2017
VIsionCare
Cataract/Anterior Segment, Retina/Vitreous
The FDA has approved a clinical study of the implantable miniature telescope (VisionCare) in AMD patients who were previously implanted with an IOL.
The implant is already FDA approved and available to patients aged 65 and older who have not had cataract surgery. In the new study, the IOL will be exchanged for the implantable miniature telescope.
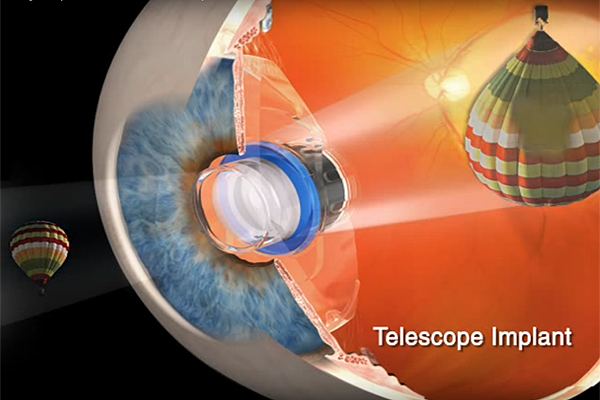
Intended for patients with profound vision impairment (BCVA 20/160 to 20/800) caused by bilateral central scotomas associated with end-stage AMD, the device renders enlarged central vision images over perimacular areas to improve central vision, while the non-operated eye provides peripheral vision for mobility and orientation.
The mini telescope is housed in a prosthetic device composed of 3 primary components: a glass capsule that contains wide-angle micro-optical elements, a clear polymethylmethacrylate (PMMA) carrier, and a blue PMMA light restrictor. The sealed optical component is snap-fitted into the carrier plate.
“Cataract surgery is often performed on patients living with macular degeneration in the hopes that an IOL will improve contrast and light. However, studies show that patients who progress to end-stage macular degeneration do not experience an appreciable improvement in their visual acuity, post cataract surgery,” said Stephen Lane, MD. “The long-term efficacy of the telescope implant in improving vision and quality of life in AMD patients has been demonstrated during studies that followed subjects up to 8 years post-surgery. This study will inform us about the safety, effectiveness, and the appropriate surgical technique for implanting the telescope in patients who have had cataract surgery before.”