JUN 30, 2017
By Keng Jin Lee
Tohuku University, Nature Scientific Reports
Comprehensive Ophthalmology, Retina/Vitreous
A group of multidisciplinary scientists from Tohoku University in Japan has built an elegant but simple microfluidic model of ocular fundus tissue to simulate the mechanics of age-related macular degeneration (AMD).
While angiogenesis has been studied extensively using co-culture models, the authors believe that a microfluidic model provides a more physiologically realistic environment than static culture insert plates. Their findings were published on June 14 in Nature Scientific Reports.
To mimic the outermost structure of the retina, the research team used human retinal pigment epithelial (ARPE-19) cells, a porous membrane and human umbilical vein endothelial cells (HUVEC). These were selected to represent retinal cells, Bruch’s membrane and the choroid, respectively.
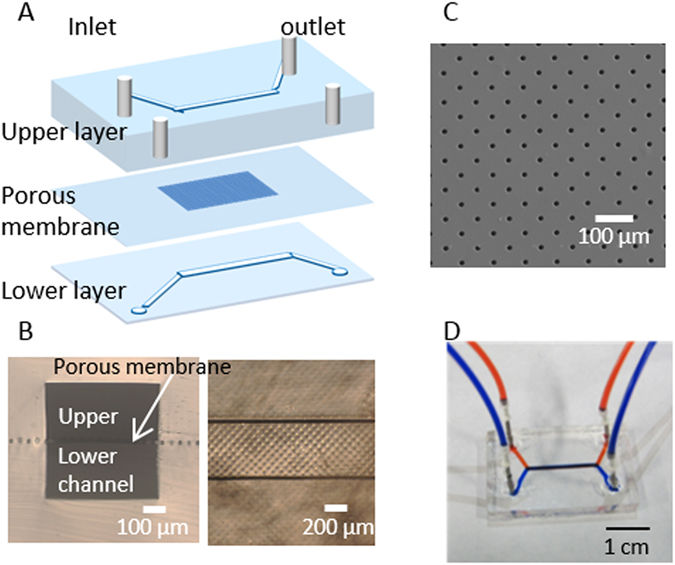
Microfluidic device configuration. (A) Exploded view and schematic. (B) Cross-section (left) and top (right) view of the device. (C) Image of the porous membrane. (D) Photograph of a device with dyes in fluidic channels.
Prior to evaluating the microfluidic co-culture device, researchers first exposed APPE-19 monocultures to low glucose and hypoxic conditions. As predicted, the cells secreted elevated levels of vascular endothelial growth factor (VEGF) compared with controls. The finding confirms previous reports that RPE cells respond to slight alterations in their microenvironment by expressing higher levels of VEGF.
Next, they assessed how epithelial cells that were under duress would affect endothelial cells within the microfluidic co-culture device. Hypoglycemic and hypoxic conditions induced a high loss of ARPE-19 cells and a concurrent increase in proliferation and directional growth of HUVECs.
The investigators hypothesize that HUVECs responded to increased VEGF levels by migrating through the membrane pores, causing detachment of ARPE-19s from their underlying surface and subsequent cell death. This partially recapitulates the neovascularization process seen in wet AMD.
Future research will assess how the co-cultures respond to other angiogenic regulators such as TNF-alpha.
According to the investigators, this organ-on-a-chip could be used for disease modeling and drug screening as an alternative to animal models. Additionally, the microdevice can be fabricated easily in a short amount of time and, with slight alterations, could be tailored to other applications.