Researchers from University of Southern California (USC) and the University of California, Santa Barbara are the first to show the feasibility of a stem cell-based implant in people with advanced dry AMD, with no adverse events noted through 1 year. Stem cell researchers from the University of California at Santa Barbara also contributed to the study.
“Our study shows that this unique stem cell-based retinal implant thus far is well tolerated, and preliminary results suggest it may help people with advanced dry age-related macular degeneration,” said co-author Mark S. Humayun, MD, director of the USC Institute for Biomedical Therapeutics and co-director of the Roski Eye Institute.
Results from the phase 1/2a trial, funded in part by the California Institute for Regenerative Medicine, appeared March 4 in Science Translational Medicine. Dr. Humayun, David R. Hinton, MD, professor of pathology at the Keck School, and Dennis O. Clegg, PhD, Wilcox Family Chair in BioMedicine and co-director of the Center for Stem Cell Biology and Engineering at UC Santa Barbara are credited as co-inventors of the implant.
The paper marks the second high-profile stem cell study in recent weeks. Last month, surgeons from Moorfields Eye Hospital in London successfully restored reading vision in 2 patients with advanced wet AMD using a specially engineered patch of retinal pigment epithelium (RPE) cells.
Both studies used human embryonic stem cell (hESC)-derived RPE cells and similar-size implants, but the researchers opted for different basement layers. Moorfields’ surgeons grew their hESC-RPE cells on a human vitronectin-coated polyester membrane, whereas the USC study used synthetic parylene. According to the authors, the parylene substrate permits RPE adhesion in a polarized monolayer and a diffusion barrier similar to Bruch’s membrane.
The 3.5 x 6.25 mm-sized implant, which housed approximately 100,000 mature, polarized, pigmented hESC-RPE cells, was delivered to 5 patients with geographic atrophy secondary to dry AMD. The procedure was successful in 4 patients.
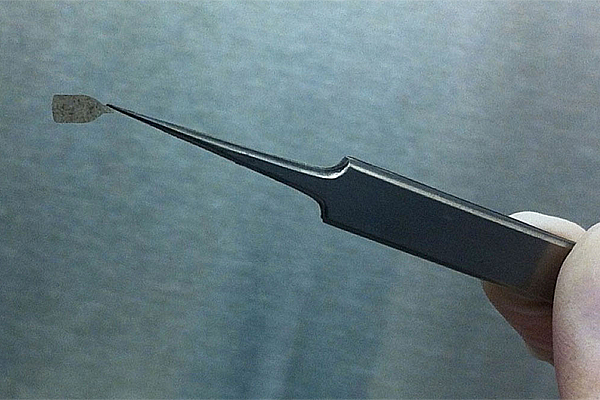
The bottle shape provides a handle to grasp the implant and a protrusion on the handle allows surgeons to know which side of the implant is facing up. Image courtesy of Britney Pennington, PhD, Center for Stem Cell Biology and Engineering, UC Santa Barbara.
Following surgery, OCT imaging showed changes consistent with hESC-RPE and host-photoreceptor integration. A preliminary assessment of the therapy’s efficacy showed that 1 eye improved by 17 letters and 2 eyes demonstrated improved fixation. Importantly, none showed progressive vision loss.
“This is the first human trial of this novel stem cell-based implant, which is designed to replace a single-cell layer that degenerates in patients with dry age-related macular degeneration,” said lead author and surgeon for the study Amir H. Kashani, MD, assistant professor of clinical ophthalmology at the Keck School of Medicine. “This implant has the potential to stop the progression of the disease or even improve patients’ vision. Proving its safety in humans is the first step in accomplishing that goal.”