Authors: Yannis M. Paulus, MD; Mark S. Blumenkranz, MD
Panretinal Photocoagulation for Treatment of Proliferative Diabetic Retinopathy
The natural course of untreated proliferative diabetic retinopathy (PDR) carries a grim prognosis. After noting retinal damage associated with gazing at a solar eclipse, Dr. Gerhard Rudolph Edmund Meyer-Schwickerath first described photocoagulation with the xenon arc photocoagulator. In this treatment, intense lesions were applied to the retina and absorbed by the retinal pigment epithelium (RPE), causing blood stasis in neovascular vessels. This treatment was limited to retinal neovascularization and was ineffective in treating neovascularization of the disc (NVD) or the posterior hyaloid face.
Subsequently, retinal laser photocoagulation was described with the ruby laser and later argon laser. Small clinical studies suggested laser photocoagulation was a promising modality for treating PDR, and the Diabetic Retinopathy Study (DRS) in the 1970s established panretinal photocoagulation (PRP) as an effective treatment for PDR.
Conventional retinal laser photocoagulation for diabetic retinopathy is typically performed with a continuous wave (cw) laser at 514 or 532 nm with exposure durations from 100 to 200 ms, spot sizes from 100 to 500 µm, and powers from 250 to 750 mW. In high-risk eyes with angiographic evidence of disc and retinal neovascularization associated with retinal capillary nonperfusion, a target of approximately 1300–1500 500 micron-sized burns spaced between one half and 1 burn width apart, beginning just outside the vascular arcades and 3 disc diameters temporal to the macula, and extending to or just beyond the equator, are typical on the temporal side of the fundus. On the nasal side of the fundus, burns begin about 1 disc diameter nasal to the optic disc and also extend to or just beyond the equator (Figure 1).
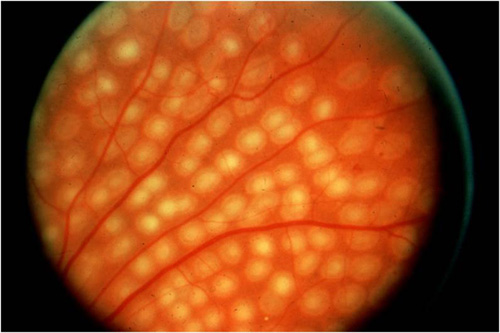
Figure 1. Appearance of PRP burns using standard technique (Diabetic Retinopathy Study/Early Treatment Diabetic Retinopathy Study).
Newer pattern scanning methods of photocoagulation (addressed later) are capable of placing more than 1 spot––typically 4 to 9 spots––essentially simultaneously with a singer press of the foot pedal. Because of the use of a scanner, shorter pulse durations in the range of 10–30 msecs rather than 100–200 msecs are used. As a result, the burns appear more regularly spaced, slightly smaller, more homogeneous in character, and less intense than traditional DRS or Early Treatment Diabetic Retinopathy Study (ETDRS) burns (Figure 2).
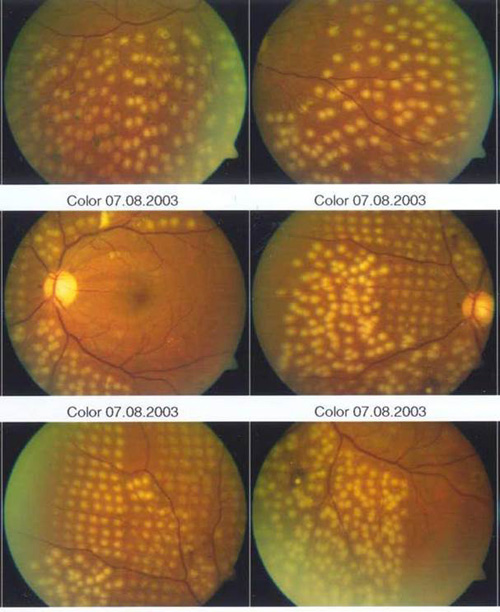
Figure 2. Side-by-side comparison of conventional and shorter-duration Pascal method burns. Conventional 100–200 msec burns have hotter white centers and are larger and more heterogeneous than 10–20 msec burns and are associated with more pain clinically.
Evidence of the Clinical Efficacy of PRP
The DRS was the seminal work establishing PRP as an efficacious treatment for PDR. The DRS was a prospective, multicenter, randomized-controlled trial sponsored by the National Eye Institute. It began in 1971 and included over 1700 patients at 15 centers. One eye of each patient was randomly assigned to immediate laser treatment with either a xenon arc or argon laser and the second eye served as a control without treatment. Treated eyes received photocoagulation to neovascularization of the retina elsewhere (NVE) and scatter photocoagulation in 1 or 2 sessions to beyond the vortex vein ampulae (the equator), with burns separated by 1 burn diameter. Argon laser treatment also involved localized treatment to NVD. Argon treatment specified 800 to 1600 spots, 500 µm burn diameter, and 100 millisecond (ms) pulse duration. Xenon arc patients were treated with 400–800 spots of 3 degrees or 200–400 spots of 4.5 degrees. Patients were included if they had a best-corrected visual acuity (BCVA) of 20/100 or better in both eyes and the presence of PDR in one eye or severe nonproliferative diabetic retinopathy in both eyes. The primary outcome measurement was severe vision loss, defined as BCVA less than 5/200 at 2 consecutive visits 4 months apart.
High risk was defined by eyes with 3 of the following risk factors: neovascularization; neovascularization within 1 disc diameter of the optic disc; NVD greater than 1/3 disc area or NVE greater than half a disc area; and preretinal or vitreous hemorrhage. In eyes with high-risk characteristics, PRP decreased the incidence of severe vision loss at 2 years from 26% of controls to 11% of treated eyes. This increased to 44% of control eyes and 20% of treated eyes at 4 years.
The DRS found that xenon arc was associated with a higher rate of complications than argon laser and thus recommended argon laser photocoagulation. The xenon arc cohort had 19% of patients lose 1 or more lines of vision and 11% of patients lose 2 or more lines, whereas argon had 11% and 3%, respectively. A quarter of xenon arc patients had peripheral field constriction, whereas less than 5% of argon laser patients did.
The British Xenon Arc Coagulation Study published in 1983 was a multicenter, randomized-controlled trial of 99 patients, where 1 eye of each patient was randomly assigned to immediate laser treatment with xenon arc and the second eye served as a control without treatment. The mean visual acuity decreased by less than 1 line in treated eyes but more than 2 lines in controls. This decrease was most significant in patients whose initial vision was 6/6 to 6/9 and was not different in those with BCVA of 6/36 or worse, thus recommending treatment in patients whose vision loss was not too far advanced.
Based on the DRS, the efficacy of treatment in patients with PDR without high-risk characteristics or NPDR was unclear. The National Eye Institute thus sponsored a randomized, multicenter clinical trial, the Early Treatment Diabetic Retinopathy Study (ETDRS), enrolling between 1980 and 1985 a total of 3711 patients with mild-to-severe nonproliferative or PDR without high-risk features who were followed at least every 4 months. The role of 650 mg daily aspirin was evaluated along with early PRP or deferral of treatment until development of high-risk characteristics. Early PRP was given either in full scatter (similar to the DRS argon with 1200 to 1600 spots, 500 µm burn diameter, and 100 ms pulse duration to the equator delivered in 2 sittings) or mild scatter (400 to 650 spots more widely spaced in 1 sitting) along with 2 macular treatments, which are described in the next section. NVE was treated directly as in the DRS. The primary outcome was severe vision loss, defined the same as the DRS with BCVA less than 5/200 at 2 consecutive visits 4 months apart, or necessitating vitrectomy. The ETDRS found a 5-year rate of severe vision loss of 2.6% in the treatment group and 3.7% of the control group. Both groups, however, had a low rate of severe vision loss, and side effects of PRP were noted in the treated group. Thus the study recommended careful follow-up without PRP for mild or moderate nonproliferative diabetic retinopathy. The ETDRS found no difference in progression to high risk or vision loss between the placebo and aspirin groups but did find a 17% decrease in cardiovascular morbidity and mortality with aspirin.
Classically, PRP was given in 2 sittings to reduce patient pain and post-laser exacerbation of macular edema. However, the DRCR Network (DRCR.net) conducted a nonrandomized, prospective, multicenter clinical trial comparing PRP in 1 session versus 4 sessions and found no clinical difference. While the central macular thickness was slightly more and vision slightly worse at 3 days and 4 weeks post-laser in 1 session, the primary endpoint of 34 weeks showed a reversal with the 4 sessions group 8 µm thicker with 2 letters of vision worse than the 1 session group.
Mechanism of Action
PDR results from angiogenic factors produced in response to retinal ischemia. While the exact mechanism for how PRP achieves its therapeutic effect is an area of active investigation, one theory as to mechanism of laser treatment is that it reduces neovascular disease by killing retinal cells in the poorly perfused portions of the retina, reducing relative ischemia, thus decreasing the production of angiogenic factors and increasing oxygenation of the vitreous. Since photoreceptors are the most metabolically active and numerous cells in the retina, PRP for PDR involves the purposeful destruction of a fraction of the photoreceptors.
Side Effects
Laser therapy is typically titrated to a visible clinical effect (graying or whitening of the retina), which corresponds to necrosis of the photoreceptors, and at higher settings, to the inner retina. Although clinically highly effective at halting angiogenesis, this can lead to unwanted side effects, including significant discomfort during application for many patients, permanent retinal scarring, and decreased peripheral, color, and night vision. Systematic clinicopathological analysis of laser-induced retinal lesions over time has demonstrated that exposures of 100 ms and longer typically produce retinal lesions that affect not only RPE and photoreceptors, but also the inner nuclear layer (INL), ganglion cell layer (GCL), and nerve fiber layer (NFL). Arcuate nerve fiber and visual field defects can result from laser lesions that affect the inner retina. PRP can also exacerbate diabetic macular edema (DME).
Permanent retinal scarring has multiple detrimental effects:
- It distorts the normal retinal architecture and replaces it with gliotic/fibrotic matrix;
- It disrupts normal retinal connectivity;
- It is associated with an infiltrative/inflammatory process involving cell loss;
- Lack of photoreceptors and/or other retinal cells in the scarring areas directly reduces the visual field sensitivity; and
- Loss of one class of retinal cells may, by trans-synaptic degeneration and by incitement of inflammation, result in loss of other retinal neurons.
Pattern Scanning Laser
Recent innovations have refined these traditional laser parameters to minimize the side effects while retaining the therapeutic effect. Semi-automated pattern scanning retinal photocoagulation has been recently introduced, which allows for patterns of 4 to 56 burns to be applied in less than 1 second using a scanning laser with shorter pulse durations. Several clinical systems are available, including the PASCAL (Topcon Corp, Santa Clara, CA, USA), the Visulas 532s VITE (Carl Zeiss Meditec, Jena, Germany), and a model by Quantel in France.
These delivery systems allow for the creation of well-aligned arrays of retinal lesions in a shorter time. Investigations of retinal photocoagulation have found that 10 to 20 ms exposures can produce retinal lesions of all clinical grades if power is increased accordingly. In addition, shorter pulse durations result in improved lesion localization, as compared to conventional 100 ms exposures. Patterned scanning laser typically utilizes settings of 532 nm wavelength, 200 µm, 20 ms, and powers from 300 to 750 mW (Figure 3).
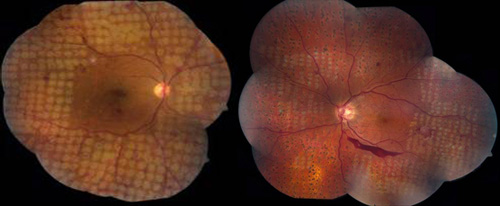
Figure 3. PRP for PDR using a pattern-scanning laser (PASCAL) method and different initial aerial spot sizes. Left: 400 micron spots. Right: 200 micron spots.
Small pilot clinical studies have been performed comparing photocoagulation with 10 to 20 ms pulses to conventional 100 ms exposures. A study by Sanghvi et al performed 10–20 ms pulse duration photocoagulation in 75 eyes in 60 patients and found it to be safe and effective.In a study by Al-Hussainy et al, 20 patients were treated with a conventional laser (100 ms, 180 mW) and short-pulse duration (20 ms, 500 mW), each lesion type in half of every treated retina. When completed, patients were asked to rate the pain of each of the 2 treatments from 0 (no pain) to 10 (worst pain ever experienced). Conventional PRP resulted in a pain score of 5.11, whereas short-pulse duration had a mean pain score of 1.4. Additional studies have confirmed that shorter duration laser burns cause less perceived pain in patients than conventional laser lesions. In a study of 17 treated eyes of 11 patients, Muqit et al evaluated the evolution of the lesions produced by 10 and 20 ms pulse duration using ophthalmoscopy, Fourier-domain optical coherence tomography (OCT), and fundus autofluorescence. Short pulse duration lesions localized to the outer retina and RPE and showed more predictable lesion characteristics at 18 months post-treatment. A retrospective study of 41 eyes treated with argon green laser before 2007, to 41 eyes treated with PASCAL after 2007, found that 73% of PASCAL-treated patients experienced persistence or recurrence of neovascularization within 6 months of treatment compared to 34% of conventional laser (p<0.0008). This study, however, gave the same number of laser spots (1438 spots in PASCAL versus 1386 spots in conventional). The decreased retinal lesion size with each PASCAL burn means that a much smaller area of the retina was treated in the PASCAL eyes. Up to 6924 PASCAL burns could be required to cause regression of severe PDR by treating equivalent areas of diseased retina in extreme cases (Figure 4).
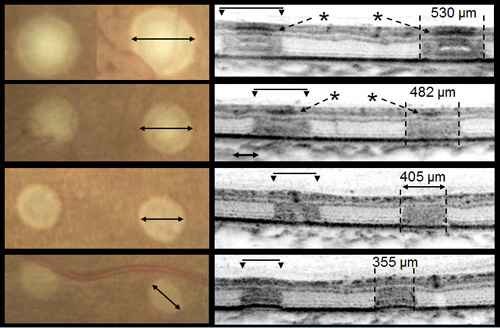
Figure 4. Differences in size and depth of acute lesion depends on pulse duration. From top to bottom: 100 ms, moderate; 20 ms, moderate; 20 mis, light; and 20 ms, barely visible. Images courtesy Daniel Lavinsky, MD.
One limitation to these studies is their small sample size. These trials occurred in cohorts with less than 100 treated eyes. While the preliminary results look encouraging due to rapidity of application, reduced perceived patient pain, and improved lesion localization, larger, multicenter randomized control trials and longer patient follow-up are needed for definitive comparison of the clinical efficacy of shorter pulse duration treatment with conventional therapy.
In conventional PRP, the endpoint of laser treatment is an ophthalmoscopically visible grayish-white lesion that develops due to thermal denaturation and coagulation of the retina. Recent evidence has suggested, however, that retinal lesions might not be permanent in smaller, less intense burns, and that the outer retina can fill the damaged areas in animal models, including rats, rabbits, and snakes. Several reports regarding light PRP, also referred to as minimum intensity photocoagulation (MIP), and subvisible treatment using micropulse photocoagulation, have indicated that these approaches may have an equivalent efficacy to conventional PRP in causing regression of high-risk PDR. Small studies find that MIP is associated with fewer complications and fewer treatment sessions. These approaches therefore could offer the therapeutic benefit of conventional therapy without its side effects (Figure 5).
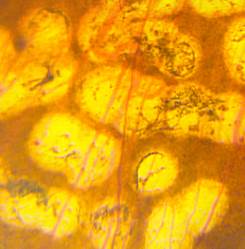
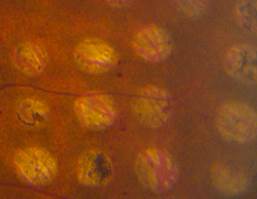
Figure 5. Comparison of late-stage burns following PRP in PDR patients. Left: Conventional burns of 100–200 mSecs result in late enlargement of burn diameter. Right: Another region from the same eye after shorter pulse duration PASCAL laser with smaller, more uniform, and less atrophic burns not subject to later enlargement.
Treatment of Diabetic Macular Edema with Laser Photocoagulation
Focal/Grid Laser
In addition to proliferative disease, diabetic retinopathy also causes vision loss through DME. In the ETDRS, eyes were categorized by the extent of retinopathy and the presence of macular edema and were randomized to receive immediate or deferred focal laser. Focal photocoagulation consisted of direct focal treatment of microaneurysms more than 500 µm from the foveal center. Treatment up to 300 µm from the foveal center was allowed if vision was 20/40 or worse. Grid photocoagulation was applied to areas of diffuse leakage and capillary nonperfusion on fluorescein angiography. Focal laser settings were 50 to 100 µm spot size, 50 to 100 ms pulse duration, and power titrated to whiten the microaneurysm. Grid laser settings were 50 to 200 µm spot size, 50 to 100 ms pulse duration, and power titrated to achieve mild burn intensities (Figure 6). The primary outcome was defined as "moderate vision loss" or 15 letters of ETDRS vision (3 lines). Clinically significant macular edema (CSME) was defined as any of the following:
- Retinal thickening within 500 µm of the foveal center;
- Hard exudates within 500 µm of the foveal center associated with adjacent retinal thickening; or
- Retinal thickening more than 1 disc area in size within 1 disc diameter from the foveal center.
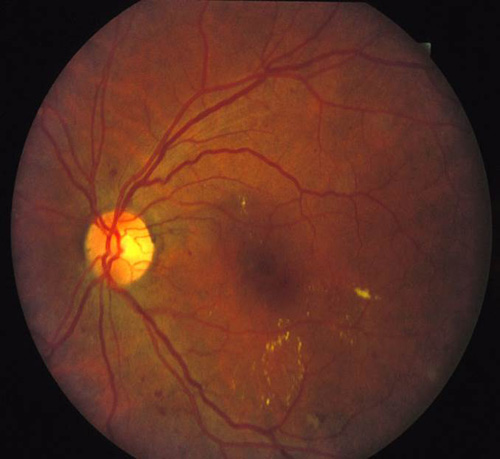
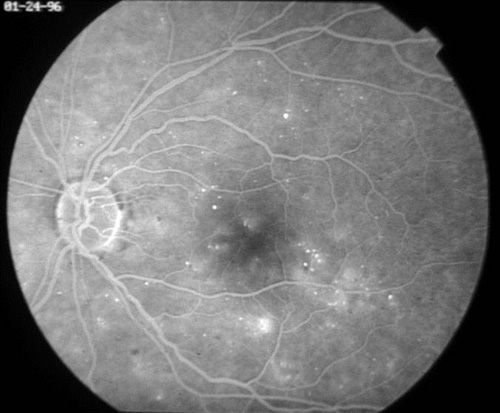
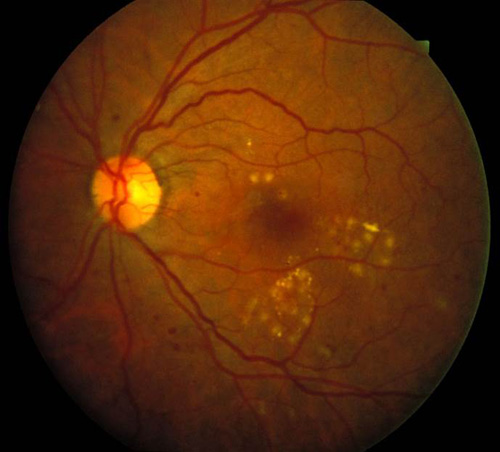
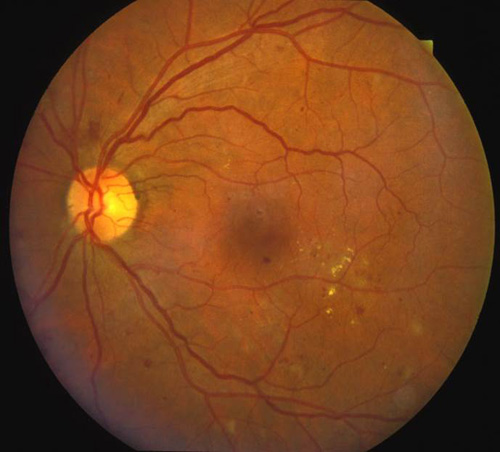
Figure 6. Top: DME before focal laser treatment. Bottom left: Immediately after. Bottom right: 3 months later. Images courtesy Harry Flynn, MD.
Eyes with macular edema that did not meet criteria for CSME did not show a benefit with focal laser, whereas eyes with CSME with central foveal involvement had a reduction in moderate vision loss from 33% in controls to 13% with focal laser. CSME without central foveal involvement had a reduction in moderate vision loss from 16% in controls to 6% with focal laser.
Since the landmark ETDRS study, clinicians have sought to continue the therapeutic benefit of focal laser with less intense laser lesions. The DRCR.net created "modified ETDRS" guidelines in which all microaneurysms are treated between 500 and 3000 µm from the foveal center with 50 µm, 50 to 100 ms lesions with power titrated to produce a grey-white burn. Grid laser is applied to all areas with edema from 500 to 3000 µm in all directions and up to 3.5 mm temporally from the foveal center with laser spots that are 50 µm, 50 to 100 ms pulse duration separated by 2 burn diameters, with power titrated to barely visible laser spots. Comparison of the modified ETDRS with a mild macular grid (MMG) in 263 patients found that at 1 year the modified ETDRS grid photocoagulation reduced central macular thickness (CMT) by 88 µm whereas MMG only reduced CMT by 49 µm. The mean visual acuity of the modified ETDRS group was stable, whereas the MMG lost 2 letters of vision at 1 year. Therefore, modified ETDRS remains the standard of care in eyes undergoing laser (Figure 7).
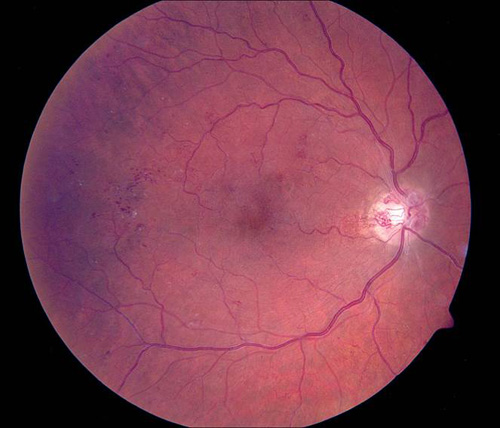
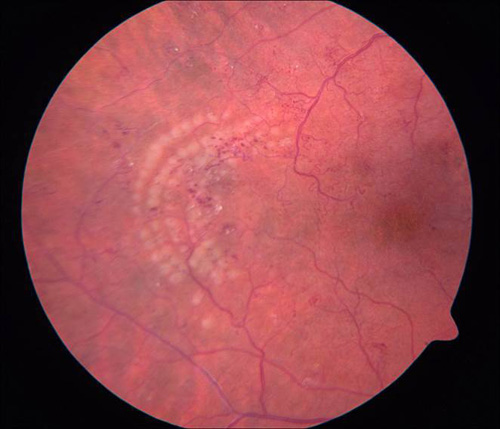
Figure 7. Grid photocoagulation before (left) and after (right) Pascal method.
Response to laser therapy has been shown to depend on subclassification of the condition. OCT can be used to subclassify DME into four categories:
- Diffuse retinal thickening (DRT);
- Cystoid macular edema (CME);
- Serous retinal detachment (SRD); and
- Vitreomacular interface abnormalities (VMIA).
In a study of 70 eyes from 45 patients who underwent focal laser photocoagulation with different subcategories of DME, patients with DRT were found to achieve a greater reduction in retinal thickness and greater improvement in visual acuity than patients with CME or VMIA.
Side Effects of Focal/Grid Laser
While effective at reducing macular edema, focal and grid laser has been shown to have some side effects. It can cause visible laser scars that can enlarge postoperatively, and it can cause complications that include choroidal neovascularization (CNV), subretinal fibrosis, and visual field loss. The recent modifications to the ETDRS have sought to use less intense laser to reduce these side effects.
New Laser Delivery Systems
There are newer laser delivery systems with clinical utility or promise in the treatment of diabetic retinopathy that involve both shorter pulse durations with limited degree of tissue injury and enhanced ergonomics, including image guidance and scanning capability.
Subthreshold Treatment
To reduce the side effects of laser therapy, particularly in the macula, groups have investigated the possibility of modifications in lesion intensity and pulse duration. In smaller studies, light laser, termed minimum intensity photocoagulation (MIP),has been associated with fewer complications and fewer treatment sessions but with similar clinical efficacy. In one study at 12 months, light focal laser was reported equivalent to classic laser in reduction/elimination of DME, visual improvement, change in contrast sensitivity, and decreased foveal thickness on OCT.
Less than barely visible lesions, including several different types of subvisible laser, have been investigated. These include: selective retinal therapy (SRT), transpupillary thermotherapy (TTT), and subvisible diode micropulse (SDM) photocoagulation, both with and without scanning systems.
Selective Retinal Therapy
One method to provide retinal phototherapy without permanent damage is selective treatment of the RPE by decreasing the laser pulse duration to the microsecond domain, termed selective retinal therapy (SRT). It has been shown that the application of microsecond pulses at or below the thermal relaxation time of melanosomes in the RPE (1 µs) leads to the formation of microbubbles and selective RPE cell death, without damaging the photoreceptors, neural retina, or choroid.Subsequent RPE proliferation and migration restores RPE continuity.
The first RPE-selective retinal laser treatment was achieved using 5 µs argon laser pulses at 514 nm and a repetition rate of 500 Hz. Currently, several microsecond pulsed laser systems are available. In an alternative SRT approach, based on a rapidly scanning continuous wave laser, RPE cells are exposed to laser light for several microseconds.
Transpupillary Thermotherapy
Another approach to nondamaging retinal treatment is transpupillary thermotherapy (TTT). In a more intense, destructive modality, it is occasionally used for the treatment of choroidal melanomas. TTT involves long exposures (~60 s) of a large spot (1.2–3 mm) at low irradiance (~10 W/cm2), using a near-infrared (NIR – 810 nm) laser.
Subvisible Diode Micropulse Photocoagulation
Shorter bursts of near infrared (NIR) radiation (810 nm) with small spot sizes (100–200 µm) have also been applied for nondamaging retinal phototherapy. Termed subvisible diode micropulse (SDM) photocoagulation, it uses the OcuLight SL clinical system (Iridex Corporation, Mountain View, CA, USA). Mathematical models have demonstrated that hyperthermia in this approach does not exceed the threshold of cell toxicity and has a high degree of selectivity. SDM allows for complete and confluent coverage of the entire diseased retina, such as the areas of macular thickening constituting DME.The 810 nm wavelength is selected due to its reduced absorption by photoreceptors and hemoglobin, and thus more selective absorption by melanin in RPE and pigmented choroid. Typically, a sequence of micropulses of 100 μs in duration separated by 50-150 μs is applied during 200 to 500 ms longer pulse envelopes (Figures 8 and 9).

Figure 8. A variety of FDA-approved units with different wavelengths and micropulsing capabilities are available. Use of ultrashort (micro- and nanosecond) pulse technology limits damage to the RPE and spares the neural retina.
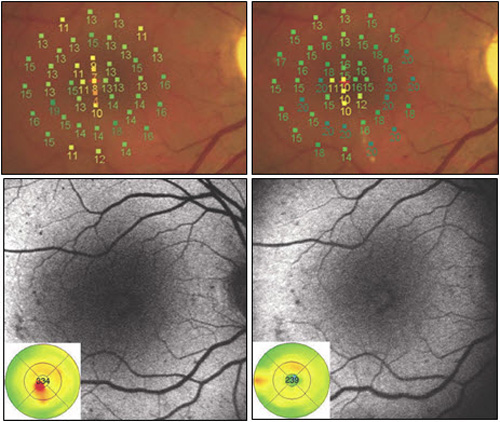
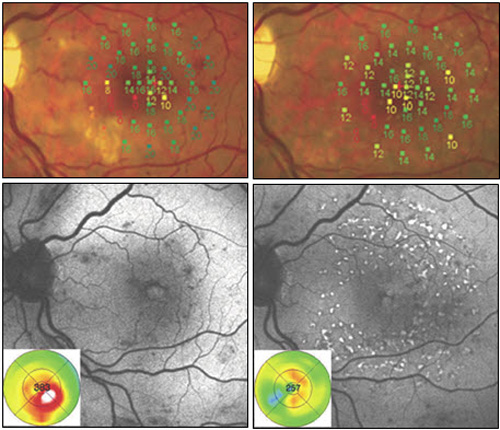
Figure 9. Recent studies have compared modified ETDRS and micropulse subthreshold grids. After 1 year (right images), both treatments were found equally effective in stabilizing BCVA and reducing central retinal thickness. With fundus AF there were no signs of laser lesions in the subthreshold treated eyes whereas spots of hyperautofluorescence identified laser-burns in modified ETDRS-treated eyes. Significant difference was found in retinal sensitivity at microperimetry: increased in subthreshold group and decreased in modified ETDRS group (P = 0.04 at 4º and P < 0.0001 at 12º).
Mechanism of Action of Sublethal Laser
The mechanism of action of laser therapy is an area of active investigation and a constellation of local cytokines is thought to be playing important roles, including VEGF, pigment epithelial-derived factor (PEDF), matrix metalloproteinases (MMP), and tissue inhibitor of matrix metalloproteinases (TIMP).It has been proposed that laser treatment results in an increase in free-radical activity by direct thermal injury or oxygen reperfusion, and it has been shown that laser injury creates a surge in free radicals.The MMP (specifically, MMP-9 and alpha2M) and transforming growth factor-beta 2 (TGF-β2) have also been noted to increase dramatically in activity after laser application. Angiostatin has been noted to modulate the therapeutic effects of laser. Recent experiments looking at rat models of hypoxia have found that photoreceptors act as a repository of growth factors of hypoxic conditions that cause angiogenesis and increased vascular permeability (Figure 10).
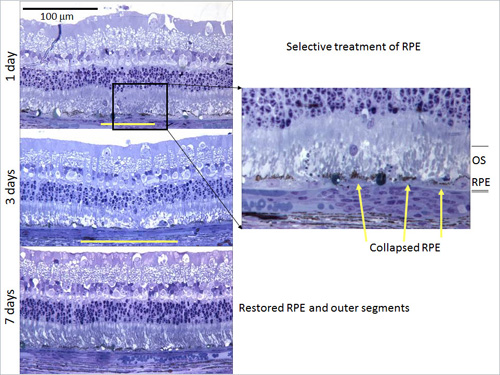
Figure 10.
Heat shock proteins (HSPs) are another group of indicators of cellular response to stress. HSPs assist in the refolding of denatured proteins as chaperone proteins. They also inhibit inappropriate protein aggregation, traffic target proteins for degradation or repair, and stabilize the cytoskeleton to maintain cell structure. HSPs are expressed at low levels at baseline, but rapidly increase expression in states of thermal, ischemic, and oxidative stress. HSPs are considered a significant component in conferring thermotolerance to heated tissue.
HSPs also act on the apoptotic and inflammatory pathways. They interfere with both caspase-dependent and caspase-independent apoptotic cascades in various tissue types, including neural tissue such as retinal ganglion cells. HSP70 upregulates the anti-apoptotic protein Bcl-2, and may also prevent mitochondrial cytochrome c release. HSP70, one particular HSP that is 70 kD in size, prevents formation of the complex central to caspase-dependent apoptosis, inhibits caspase-3 activation, and interferes with the caspase-independent apoptosis inducing factor (AIF). HSP possibly interacts with NFkB, a transcription factor associated with genes involved in inflammation, and its inhibitor IkB to reduce TNF-a.
HSP expression has been demonstrated in response to laser treatment in the choroid and retina of rabbit and rodent models. It has been found that laser treatment in mice at half the threshold power of RPE damage (100 ms, 532 nm, 400 µm diameter) induced transcription of HSP70, an indication of cellular response to sublethal thermal stress.
Clinical Evidence for Sublethal Laser
In 2000, the first clinical study reported the efficacy of SRT in 12 patients with diabetic maculopathy, 10 patients with soft drusen, and 4 patients with central serous retinopathy. SRT was performed with an Nd:YLF laser (wavelength of 527 nm) using a 1.7 µs pulse duration. The number of hard exudates, drusen, and serous detachment improved after treatment. Microperimetry confirmed no visual loss after treatment. Preliminary results from an international multicenter trial appear quite promising among 60 patients with diabetic maculopathy.
In a retrospective review of 77 patients with occult CNV, TTT has been shown to result in visual stabilization (47%) or improvement (22%) in the majority of patients. Other retrospective reviews have similarly shown a closure of approximately 75% of classic and occult membranes in CNV in patients after TTT treatment. Concerns, however, have been raised about occasional retinal damage resulting from TTT therapy.
Subvisible diode micropulse has also been thought to be clinically effective in the treatment of DME and PDR in small clinical series. SDM has been judged as effective as modified ETDRS laser photocoagulation in the treatment of CSME without producing visible retinal damage. Beneficial effects in the treatment of neovascularization and macular edema have been recorded in clinical exams and by retinal thickness measurements with OCT. SDM has been found effective for the treatment of macular edema from venous occlusion and diabetic retinopathy. These studies, however, are limited by their size and duration of the patient follow up.
Further advances have recently been made in the development of both semi-automated lasers, including PASCAL with Endpoint Management (Figure 11). More fully automated lasers, including the NAVILAS system, incorporate elements of image acquisition and management, as well as eye-tracking capability. Treatment sessions can be planned out in advance on a graphics user interface, and the laser can focally treat prespecified lesions with a high degree of accuracy and reproducibility.
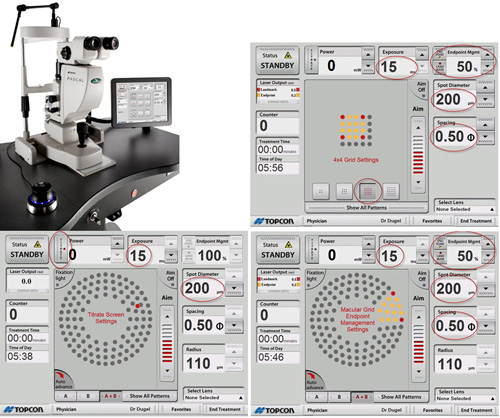
Figure 11. Endpoint management GUI.
Suggested Reading
- Cullen JF, Town SM. Diabetic retinopathy. Review of 82 patients presenting with unilateral blindness. Trans Ophthalmol Soc U K. 1975; 95(4):484-486.
- Kapany NS, Peppers NA, Zweng HC, Flocks M. Retinal photocoagulation by lasers. Nature. 1963;199:146-149.
- Little HL, Zweng HC, Peabody RR. Argon laser slit-lamp retinal photocoagulation. Trans Am Acad Ophthalmol Otolaryngol. 1970;74(1):85-97.
- Diabetic Retinopathy Study Research Group. Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Invest Ophthalmol Vis Sci. 1981;21(1 Pt 2):1-226.
- Diabetic Retinopathy Study Research Group Indications for photocoagulation treatment of diabetic retinopathy: Diabetic Retinopathy Study Report no. 14. Int Ophthalmol Clin. 1987;27(4):239-253.
- Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98 (5 Suppl):766-785.
- Brucker AJ, Qin H, Antoszyk AN, et al.; Diabetic Retinopathy Clinical Research Network. Observational study of the development of diabetic macular edema following panretinal (scatter) photocoagulation given in 1 or 4 sittings. Arch Ophthalmol. 2009;127(2):132-140.
- Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia. 2000;43(11):1404-1407.
- Jennings PE, MacEwen CJ, Fallon TJ, Scott N, Haining WM, Belch JJ. Oxidative effects of laser photocoagulation. Free Radic Biol Med. 1991;11(3):327-330.
- Birnbach CD, Jarvelainen M, Possin DE, Milam AH. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101(7):1211-1219.
- Al-Hussainy S, Dodson PM, Gibson JM. Pain response and follow-up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye. 2008;22(1):96-99.
- Muqit MMK, Gray JCB, Marcellino GR, et al. Fundus autofluorescence and Fourier-domain optical coherence tomography imaging of 10 and 20 millisecond Pascal retinal photocoagulation treatment. Br J Ophthalmol. 2009;93(4):518-525.
- Muqit MM, Marcellino GR, Henson DB, Young LB, Turner GS, Stanga PE. Pascal panretinal laser ablation and regression analysis in proliferative diabetic retinopathy: Manchester Pascal Study Report 4. Eye. 2011;25(11):1447-1456.
- Bandello F, Brancato R, Menchini U, et al. Light panretinal photocoagulation (LPRP) versus classic panretinal photocoagulation (CPRP) in proliferative diabetic retinopathy. Semin Ophthalmol. 2001;16(1):12-18.
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796-1806.
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987;94(7):761-774.
- Kim NR, Kim YJ, Chin HS, Moon YS. Optical coherence tomography patterns in diabetic macular oedema: prediction of visual outcome after focal laser photocoagulation. Br J Ophthalmol. 2009;93(7):901-905.
- Morgan CM, Schatz H. Atrophic creep of the retinal pigment epithelium after focal macular photocoagulation. Ophthalmology. 1989;96(1):96-103.
- Lewen RM. Subretinal neovascularization complicating laser photocoagulation of diabetic maculopathy. Ophthalmic Surg. 1988;19(10):734-737.
- Rutledge BK, Wallow IH, Poulsen GL. Sub-pigment epithelial membranes after photocoagulation for diabetic macular edema. Arch Ophthalmol. 1993;111(5):608-613.
- Hudson C, Flanagan JG, Turner GS, Chen HC, Young LB, McLeod D. Influence of laser photocoagulation for clinically significant diabetic macular oedema (DMO) on short-wavelength and conventional automated perimetry. Diabetologia. 1998;41(11):1283-1292.
- Sinclair SH, Alaniz R, Presti P. Laser treatment of diabetic macular edema: comparison of ETDRS-level treatment with threshold-level treatment by using high-contrast discriminant central visual field testing. Semin Ophthalmol. 1999;14(4):214-222.
- Roider J. Laser treatment of retinal diseases by subthreshold laser effects. Semin Ophthalmol. 1999;14(1):19-26.
- Roider J, Michaud NA, Flotte TJ, Birngruber R. Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol. 1992;110(12):1786-1792.
- Roider J, Brinkmann R, Wirbelauer C, Laqua H, Birngruber R. Retinal sparing by selective retinal pigment epithelial photocoagulation. Arch Ophthalmol. 1999;117(8):1028-1034.
- Aaberg TM, Jr., Bergstrom CS, Hickner ZJ, Lynn MJ. Long-term results of primary transpupillary thermal therapy for the treatment of choroidal malignant melanoma. Br J Ophthalmol. 2008;92(6):741-746.
- Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2006;37(5):370-377.
- Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511-524.
- Foulds WS, Kaur C, Luu CD, Kek WK. A role for photoreceptors in retinal oedema and angiogenesis: an additional explanation for laser treatment? Eye. 2010;24(5):918-926.
- Lanneau D, de Thonel A, Maurel S, et al. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1(1):53-60.
- Franklin TB, Krueger-Naug AM, Clarke DB, et al. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia. 2005;21(5):379-392.
- Desmettre T, Maurage CA, Mordon S. Transpupillary thermotherapy (TTT) with short duration laser exposures induce heat shock protein (HSP) hyperexpression on choroidoretinal layers. Lasers Surg Med. 2003;33(2):102-107.
- Brinkmann R, Roider J, Birngruber R. Selective Retina Therapy (SRT): A Review on Methods, Techniques, Preclinical and First Clinical Results. Bull Soc Belge Ophtalmol. 2006;(302):51-69.
- Newsom RS, McAlister JC, Saeed M, McHugh JD. Transpupillary thermotherapy (TTT) for the treatment of choroidal neovascularisation. Br J Ophthalmol. 2001;85(2):173-178.