Corneal Collagen Cross-linking Approved to Treat Keratoconus in U.S.
People with keratoconus have a new treatment option. The U.S. Food and Drug Administration (FDA) has approved corneal collagen cross-linking (CXL) for progressive thinning and distortion of the cornea.
The treatment uses a special laser and eye drops to promote “cross-linking,” or strengthening, of collagen fibers in the cornea.
CXL has been in wide use outside the United States for about a decade. Based on three year-long studies, the FDA approved the new treatment for keratoconus and other types of corneal ectasia. Corneal ectasia is the general name for conditions in which the cornea gets thinner and changes shape.
The cornea is the clear front window of the eye where light enters. It helps you see by focusing the light.
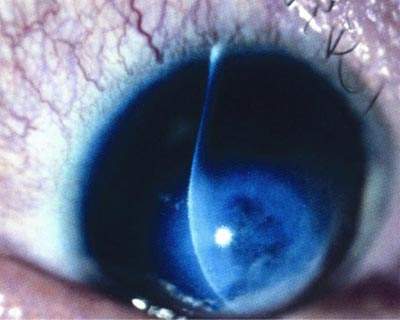
Close up of eye with keratoconus, illuminated during exam.
The cornea is normally round and dome-like. In people with keratoconus, the cornea has too few collagen fiber cross-links. These links act as support beams. The lack of cross-links allows the cornea to develop a cone-like bulge. The condition causes blurring and distorted vision that is difficult to correct with glasses.
Some people with keratoconus experience significant vision loss, and may need corneal transplants. The condition affects one in every 2,000 Americans, according to the National Eye Institute.
The treatment approved by the FDA is made by Avedro. First, eye drops are applied, then an electronic device shines a special type of ultraviolet light on the cornea. The treatment increases the collagen cross-links, which helps prevent the cornea from bulging.
“The approval of CXL in the United States finally brings the only treatment shown to halt progression of keratoconus or corneal ectasia to thousands of Americans,” Dr. Wuquass Munir, a cornea specialist from the University of Maryland, told the Academy. “The use of CXL in the U.S. could truly change the paradigm of treatment for keratoconus and corneal ectasia, and have a marked impact on the need for keratoplasty [corneal transplant] in these patients.”
Ulcerative keratitis—a serious inflammation of the cornea—is a possible side effect of the treatment.
Avedro said the new treatment should be available through ophthalmologists before the end of 2016.