By Sidney M. Gospe, III, MD, PhD; M. Tariq Bhatti, MD; Mays A. El-Dairi, MD
Introduction
Optical coherence tomography (OCT) is a noninvasive imaging technology that uses near-infrared waves and interferometry to produce in vivo tomograms (optical cross-sections).1 The tissue being imaged does not require direct contact with the OCT instrument, but it is essential that there be a clear intervening media that will not absorb or scatter the waves. Hence, OCT is best suited to study external structures (eg, skin and teeth), as well as internal structures that may be directly approached using minimally invasive endovascular or endoscopic techniques (eg, cardiovascular and gastrointestinal tissues).2 By far, the most widely adopted use of this technology has been in imaging ocular tissues, with OCT having revolutionized the field of ophthalmology as an indispensable tool for diagnosing a wide variety of posterior and anterior segment diseases.3-6
OCT has become an invaluable technology in ophthalmology due to its ability to produce representations of ocular structures with near-histologic axial resolution and to delineate and quantify the thicknesses of various tissue layers. Qualitative inspection of OCT scans and quantitative analysis with integrated software allow the ophthalmologist to identify microscopic ocular pathologies that may not be apparent on clinical examination and to assess their stability over time. It is important to note, however, that while OCT can uncover abnormalities in ocular anatomy, it does not directly assess the patient’s visual function—any relationship between OCT findings and abnormal ocular physiology can only be inferred. Furthermore, OCT has some important practical limitations. First, there is a requirement for clear ocular media, as image quality is severely degraded by ocular surface irregularities, cataract, and inflammation or hemorrhage in the anterior chamber of vitreous. OCT images are also subject to artifacts from eye movement, operator-dependent scan quality, and tracing errors that may result in inaccurate thickness measurements. Fortunately, technological improvement in recent years has mitigated some of these limitations. While the earliest commercial form of OCT, time domain-OCT (TD-OCT), was limited by relatively slow acquisition times and poor resolution, the advent of spectral domain OCT (SD-OCT) has provided superior axial image resolution (now 3-15 microns) and much faster scan acquisition, which significantly reduces artifacts from eye motion and improves work-flow in the clinic.7 The recent development of swept-source OCT (SS-OCT) further improves acquisition time and tissue penetration and will likely be integrated into the clinical setting in the coming years.8 Improvements in software and more intuitive interface design make proper centration of images less operator-dependent and longitudinal OCT comparisons more reliable.
Adoption of OCT into clinical practice has been slow in pediatric ophthalmology, yet its objective and quantitative nature would seem to hold particular advantages for this specialty. This is especially true for treating patients with suspected optic nerve disease. Diagnosis and monitoring of optic neuropathies in the pediatric population by clinical means can be a significant challenge when compared to that of adult patients. Detection of abnormalities in visual acuity, visual fields, and color vision in children may be of limited accuracy in infants, young children with short attention spans or poor cooperation, and older children with cognitive/developmental comorbidities. Furthermore, ophthalmoscopic findings may be subtle in some pediatric optic neuropathy cases, making it challenging to distinguish between pathology of the retina, optic nerve, or central nervous system (CNS) as the etiology of poor vision. While ancillary testing such as neuro-imaging or electrophysiologic studies like electroretinography and visual evoked potentials have played important diagnostic roles in such situations, OCT may prove to be a more efficient and cost-effective alternative in many cases. In this chapter, we will review the uses of OCT that are currently being applied in pediatric optic neuropathies and discuss new applications likely to emerge in the coming years.
Relevant Anatomy and OCT Imaging Protocols
The optic nerve connects the neurosensory retina to the CNS, transmitting light-evoked electrical signals that ultimately result in image formation, pupillary response, and entrainment of the circadian rhythm. The optic nerve comprises the axons of retinal ganglion cells (RGCs), third-order neurons that reside in the inner retina and receive signals from photoreceptors of the outer retina via second-order neurons such as bipolar cells and amacrine cells. RGC axons course along the retinal nerve fiber layer (RNFL), the innermost layer of the retina directly abutting the vitreous cavity, to converge at the optic nerve head (ONH), where they represent the major constituent of the neuroretinal rim of the ONH. Once the RGC axons penetrate the sclera via the lamina cribrosa, they become myelinated by oligodendrocytes, allowing for rapid saltatory conduction of electrical signals along the retrolaminar optic nerve to the CNS.
In the evaluation of optic neuropathies, OCT can provide valuable data about the microanatomy of the ONH, the RNFL, and the RGCs and other cellular layers of the retina. There are multiple imaging protocols that may be employed to optimally evaluate these different structures.
High-Resolution Cross-Sectional Retinal Scans
The posterior segment of the eye is made up of concentrically layered tissues, with the sclera surrounding the vascular choroid, which in turn envelops the retinal pigment epithelium (RPE) and the neurosensory retina. The retina and choroid are themselves layered tissues. Each of these biological layers has distinct reflective properties and therefore can be delineated on OCT (Figure 1A). Cross-sectional scans using SD-OCT allow for exceptional visualization of the various layers and measurement of their thicknesses. These 2-dimensional images are analogous to B-scans obtained with ultrasonography, but in the case of OCT, greatly superior axial resolution comes at the expense of reduced tissue penetration, such that the sclera and orbital tissues posterior to the globe are not well imaged.
Within the retina, different neuronal populations are known to localize to different layers. The photoreceptors are found in the outer retina, abutting the RPE. The nuclei of the photoreceptors reside in the outer nuclear layer (ONL), while the junction of their inner segment and outer segment compartments roughly corresponds to the ellipsoid zone (EZ) layer on OCT. In the outer plexiform layer (OPL), photoreceptors synapse with second-order neurons, whose nuclei are found in the inner nuclear layer (INL). These cells in turn synapse at the inner plexiform layer (IPL) with the RGCs, whose nuclei form the ganglion cell layer (GCL) and whose axons comprise the RNFL and ultimately the optic nerve. An optic neuropathy would therefore be expected to result in abnormalities (thinning or discontinuities) of the IPL, GCL, or RNFL layers on OCT. As discussed later in this chapter, OCT abnormalities in other retinal layers have occasionally been described in patients with definitive optic neuropathies.
Macular Map
OCT software can be used to generate macular maps that integrate multiple cross-sectional macular scans. The result is a 3-dimensional topographical map, typically centered on the foveal depression (Figure 1B). The overall thickness of the retina can be quantified and is commonly reported as average thickness values in 3 concentric rings. The foveal area corresponds to the innermost 1 mm diameter circle, while the inner ring has a 3 mm diameter, and the outer ring a 6 mm diameter. Abnormally thick retinal volumes may arise from retinal edema or subretinal fluid, while abnormally thin volumes are seen with retinal atrophy. It is possible to follow patients longitudinally by obtaining serial OCT scans and comparing macular volumes over time. More recently, software has been developed to automatically segment each retinal layer, so that the average thickness of individual layers may be quantified. For instance, the thickness of the combined GCL + IPL has shown promise in assessing the severity and progression of optic neuropathies.
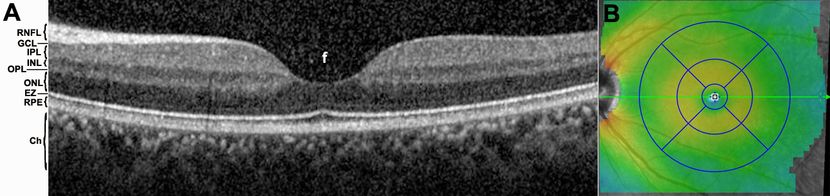
Figure 1. OCT imaging of the macula. (A) Cross-sectional scan through a normal macula, centered on the foveal depression (f). Many layers of the posterior segment are easily discernible on SD-OCT imaging, including the choroid (Ch), retinal pigment epithelium (RPE), ellipsoid zone (EZ), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), and retinal nerve fiber layer (RNFL). (B) Macular map. The thickness of the retina is depicted by color scale, while 3 concentric circles centered on the foveal depression with diameters of 1, 3, and 6 mm, are used to report retinal volume and average retinal thickness values for each sector.
Enhanced Depth Imaging
Enhanced depth imaging (EDI) compensates for the poor penetration of the near-infrared waves used for routine OCT imaging in order to optimize visualization of structures deep to the retina. It involves bringing the OCT system closer to the subject’s eye such that the line of highest resolution is centered on the outer retina/RPE junction, rather than on the vitreous/inner retina junction, as it is in traditional OCT imaging.9 EDI allows for better imaging of the choroid and the retrolaminar optic nerve.10
Retinal Nerve Fiber Layer Scan
Quantitative analysis of the RNFL was first widely used by glaucoma specialists to follow the progression of glaucomatous optic neuropathies.11 This analysis is performed by obtaining a circular scan at a diameter of 3.4-3.5 mm that is centered on the ONH (Figure 2). The axons of virtually all RGCs will pass through this circular zone before converging at the ONH and will therefore be captured in this analysis. The scan is segmented, and the thickness of the peripapillary RNFL is measured along the circumference of the circle and reported by sector, along with the average RNFL thickness overall. These values are compared to a normative database to determine whether the overall peripapillary RNFL or particular sectors of it are thinner or thicker than those in a given percentage of the population. Focal RNFL thinning on OCT is known to correlate well with RNFL defects and neuroretinal rim thinning observed on ophthalmoscopy that are characteristic of glaucomatous damage.12-14 Longitudinal comparisons of the RNFL maps in a single patient can argue for disease stability or progression and provide invaluable information for tailoring treatment to the individual patient.15 In light of its utility in glaucoma, this technology has now been adopted to study optic nerve pathology from various other etiologies including optic neuritis,16-20 compressive optic neuropathies,21,22 hereditary optic neuropathies,23-25 and others.26,27
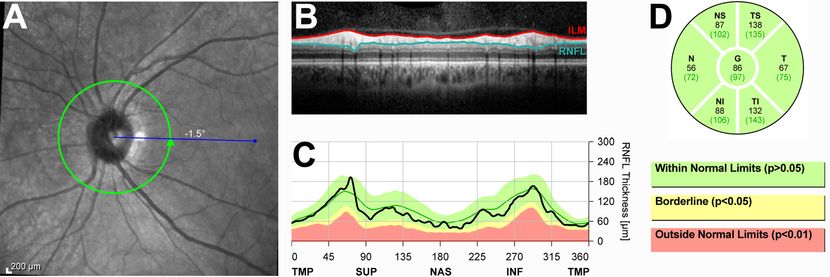
Figure 2. Normal OCT of the retinal nerve fiber layer (RNFL). (A) RNFL thickness is measured along the circumference of the 3.5 mm circle centered on the optic nerve head. (B) Circumferential OCT scan of the peripapillary retina along the circle depicted in (A). The RNFL is the top, highly reflective layer of the retina. (C) Quantification of RNFL thickness plotted against the position along the peripapillary OCT scan (in degrees). The RNFL is typically thickest in the superior (SUP) and inferior (INF) quadrants and thinner in the nasal (NAS) and temporal (TMP) quadrants. For easy comparison, the plot overlies a color-coded area demonstrating the normal variation of RNFL thicknesses taken from a normative database. (D) Quantification of peripapillary RNFL thickness by sector. For comparison, the mean value for the normal population is shown in parentheses for each sector. In this patient, all sectors have an average RNFL thickness within the range encompassing 95% of subjects from the normative database.
One limitation to widespread use of RNFL measurements in pediatric patients is the lack of a normative database that can be used for comparison, as the databases currently in use represent only white patients of at least 18 years of age. It has been reported that peripapillary RNFL thickness is slightly greater in children than in adults.28 Several studies have been performed to create basic normative datasets in children,28-33 and it is expected that appropriate pediatric normative RNFL values will be incorporated into commercial OCT software in the coming years.
Optic Nerve Head Map
The ONH map is similar to the macular map but is instead centered on the optic disc (Figure 3). It may be used to quantify ONH parameters such as total diameter, the depth and diameter of the central cup, and the thickness of the neuroretinal rim,34 as well as to qualitatively assess the ONH and peripapillary area for abnormalities such as choroidal neovascularization and optic nerve pits.35,36 By using the EDI protocol on the optic nerve, one may assess the angulation of Bruch’s membrane of the peripapillary RPE and also identify buried ONH drusen,37, 38 both of which may have utility in distinguishing papilledema from pseudopapilledema.
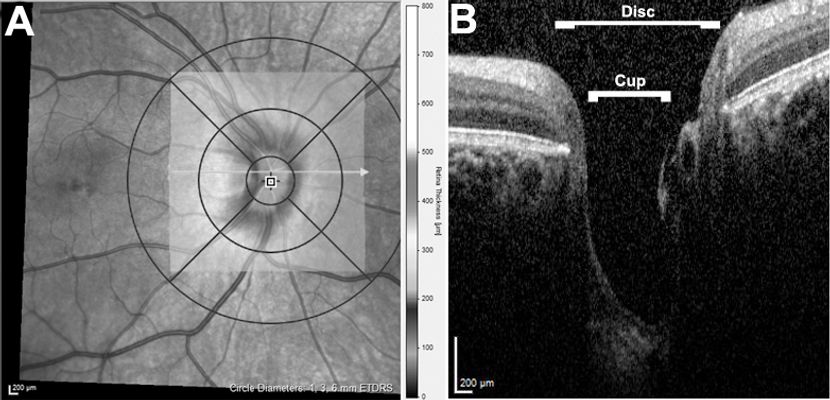
Figure 3. OCT of the optic nerve head (ONH). (A) ONH map, with the thickness of neuroretinal tissue at each point depicted according to the scale bar to the right. (B) Cross-sectional scan through the ONH. The border and contour of the ONH can be clearly assessed, as can the central cup, which in this patient is very deep.
Practical Considerations for Pediatric Patients
OCT instrumentation was designed to be employed on an ambulatory adult subject. The instrument is typically mounted on a tabletop, and the patient’s head is immobilized against a chin rest and a strap for the forehead. The patient is instructed to gaze at a fixed target in order to maintain the ocular structure of interest in stable position for image acquisition. Though this is a challenging task for a child, faster acquisition times afforded by SD-OCT7 have made it possible to obtain high-quality images in cooperative children as young as 5 years old. Furthermore, the development of eye tracking technology, which employs 2 light beams to register the position of the fundus and maintain alignment during image acquisition, increases the likelihood of obtaining a useful image from a child who has limited ability to maintain fixation.39,40 Nevertheless, because significant patient cooperation remains essential for successful image acquisition using the traditional OCT setup, and because imaging of the youngest children is not possible on a mounted unit, the recently developed handheld OCT fills an important niche in pediatric ophthalmology. The handheld unit can be used to obtain images from recumbent infants without sedation, as well as in sedated children for whom the traditional OCT setup is not feasible.41-43 Handheld OCT can thus be a useful adjunct to an examination under anesthesia in the operating room or can be employed when a child is to be sedated for a neuro-imaging study such as MRI.
Application of OCT in Pediatric Optic Neuropathies
Glaucoma
Within the field of adult glaucoma, OCT now has a long track record as a useful aid in monitoring the stability of the ONH over time in treated patients and in helping to diagnose pre-perimetric glaucoma in patients with ocular hypertension or suspicious-appearing optic nerves. In the realm of pediatric glaucoma, measurements of RNFL thickness and macular maps have also been shown to correlate well with the severity of optic nerve head cupping30,44,45 and to have reasonable reproducibility.46,47 Interestingly, when RNFL thickness was investigated in pediatric glaucoma patients who exhibited the well-described phenomenon of reversal of cupping following successful glaucoma surgery, the peripapillary RNFL remained thinned despite the healthier appearance of the ONH.48 Thus, OCT may be a more sensitive method of assessing severity of glaucomatous damage in children than the clinical examination.
OCT would seem particularly well suited for the management of pediatric glaucoma in light of the inability to obtain reliable visual fields in many of these patients, yet its adoption in this context has been very slow. As mentioned above, pediatric normative values have yet to be integrated into most commercial machines, which represents the primary limitation of the machines' usefulness in the pediatric population. It is known that mean values for these OCT parameters vary by race (blacks have thicker average and superior quadrant RNFL than whites), axial length (longer axial length is associated with thinner RNFL) and age (RNFL thickness is lower in older white children).31 Age-related changes in RNFL thickness in children might seem particularly problematic in managing pediatric glaucoma patients longitudinally. However, in a study in which OCT was repeated serially in a sample of normal pediatric patients, there was no significant change in mean RNFL thickness over time.47 It must be noted, however, that this study was limited to eight patients, and follow-up did not extend beyond a 3-year period. Regardless, unless larger future studies indicate that there is a profound thinning in RNFL thickness over the course of childhood, RNFL measurements on OCT should prove helpful to the management of pediatric glaucoma, as its utility is well established in adult glaucoma despite the known age-related decrease of RNFL thickness in adults.49
Papilledema and Pseudopapilledema
Papilledema is defined as swelling of the ONH due to elevated intracranial pressure (ICP). Elevated ICP may occur secondary to intracranial pathology such as a mass, venous sinus thrombosis, or meningitis, or it may be idiopathic (eg, idiopathic intracranial hypertension, IIH). Because the subarachnoid space within the optic nerve sheath is continuous with that surrounding the brain and spinal cord, the pressure of the cerebrospinal fluid (CSF) is transmitted directly to the optic nerve as it exits the globe posterior to the lamina cribrosa. Elevations in ICP can thus have a compressive effect on the axons and vasculature of the optic nerve, producing significant clinical manifestations. Elevated ICP reduces the rate of axoplasmic flow within the optic nerve, causing organelles and cytoplasm to accumulate within RGC axons anterior to the lamina cribrosa, resulting in clinically apparent swelling.50 As a result, the neuroretinal rim of the ONH, which usually has a sharp border on ophthalmoscopy, becomes elevated with indistinct margins. Due to the absence of a muscular wall, the central retinal vein is more subject to compression than the central retinal artery as it passes through the substance of the optic nerve while exiting the globe. Therefore, elevated ICP can cause venous stasis, resulting in dilation and tortuosity of the retinal veins and occasionally peripapillary retinal hemorrhages. In most cases, papilledema can be successfully treated without residual visual deficit, but cases of fulminant or chronic papilledema may lead to optic atrophy and permanent visual field defects or reduced visual acuity.
OCT is not yet considered standard of care in evaluating and managing patients with papilledema, but recent publications have demonstrated the promise of the technology in this setting. The ONH map protocol with automated software segmentation can provide a total disc volume, which is markedly increased in papilledema.51 Axoplasmic stasis is not limited to the ONH itself, as the surrounding retina and particularly the peripapillary RNFL also become swollen with increasing severity of papilledema. Indeed, quantitative RNFL scanning protocols have become the most popular OCT application for evaluating patients with suspected papilledema.52 A blinded study demonstrated that OCT measurement of peripapillary RNFL thickness and total retinal thickness compared favorably to expert review of optic disc photographs when grading the severity of papilledema.53 An additional intriguing potential use of OCT is to obtain linear scans through the ONH to assess the angle of Bruch's membrane in the papillary area (Figure 4). This hyperreflective band lying between the RPE and choroid typically has a slightly negative angulation (ie, angling away from the vitreous cavity) as it passes into the ONH. However, in two-thirds of patients with papilledema, the angle was shown to be positive, compared to only 2% of normal patients,54 suggesting a mechanical deformation produced by elevated ICP (essentially a correlate of the flattened posterior sclera often observed on MRI in patients with papilledema).
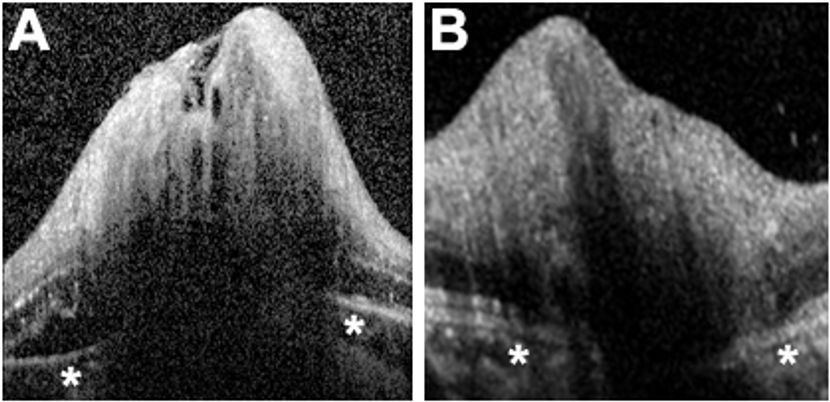
Figure 4. Assessment of Bruch’s membrane angulation on ONH scan. (A) ONH scan of a patient with papilledema. Note that underneath the swollen ONH, Bruch’s membrane and the RPE (overlying the asterisks) demonstrate a positive angulation (angling upward toward the vitreous). (B) ONH scan of a patient with optic disc swelling unrelated to elevated ICP (optic neuritis, in this case). Bruch’s membrane and the RPE demonstrate a negative angle (away from the vitreous).
In many cases, the greatest value of OCT may not be in the initial diagnosis of papilledema (which is often readily apparent on ophthalmoscopy), but in monitoring the response of patients to treatments like CSF diversion procedures or diuretics for IIH. Quantitative changes in RNFL thickness or Bruch’s membrane angulation have been shown to correlate well with changes of clinical severity of papilledema over time.55, 56 In pediatric patients in whom visual acuity and visual field testing is not sufficiently reliable to make therapeutic decisions, objective OCT data may prove particularly helpful. However, this must be cautiously interpreted and not assumed that decreasing RNFL thickness is necessarily a reassuring sign of a response to treatment, but potentially a sign of optic atrophy, which can lead to RNFL thinning and poor visual outcome in patients with papilledema.57
Macular maps can also be useful in evaluating papilledema. A recent report demonstrated that a thinned GCL-IPL complex below 70 µm on presentation or a decrease in thickness by 10 µm over 2-3 weeks correlates with irreversible vision loss in patients with IIH.58 Though papilledema is primarily a disease of the optic nerve and RGCs, OCT abnormalities in other retinal layers of the macula may be seen in patients with severe papilledema.59 Particularly interesting is the observation that changes in the parafoveal EZ layer may persist following resolution of papilledema in children with IIH 57 (Figure 5). These findings, which are suggestive of damage to retinal photoreceptors, are associated with permanent visual dysfunction, and were also observed on OCT in the acute phase of papilledema. While additional investigation is required, it is possible that OCT findings on the initial evaluation of patients with papilledema may allow for better risk stratification and aid the clinician in determining how aggressively to treat IIH (ie, proceeding immediately with surgery versus starting conservatively with medicine).
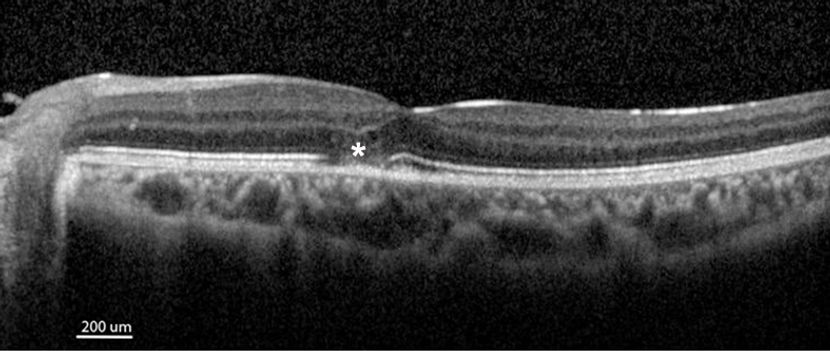
Figure 5. Outer retinal damage following severe papilledema. OCT of the macula after resolution of papilledema in a child treated for severe idiopathic intracranial hypertension. Though the ONH and RNFL swelling have subsided, the patient demonstrates a persistent focal discontinuity of the ellipsoid zone (asterisk), consistent with irreversible damage to parafoveal photoreceptors.
A related potential application of OCT that has garnered significant interest is in distinguishing papilledema from pseudopapilledema. Pseudopapilledema may be the result of physiologic small crowded discs, epipapillary glial tufts, vitreopapillary traction, or buried optic disc drusen.60 The need to distinguish between papilledema and pseudopapilledema often arises when abnormal optic discs are noted incidentally in children undergoing their first routine dilated fundus exam, and it may be a particular diagnostic challenge when a child endorses the common complaint of headache. Definitively excluding true papilledema in the clinic would be particularly desirable for pediatric ophthalmologists in order to avoid subjecting children to unnecessary neuro-imaging studies and lumbar punctures.
Initial studies investigating the value of OCT imaging in this setting have been inconclusive. Reports of peripapillary RNFL thickness measurements have been inconsistent with regard to whether there is a significant difference between patients with papilledema and those with pseudopapilledema due to congenitally crowded nerves 61-63 or buried drusen.37, 64 It has been reported that peripapillary total retinal volume in the ring between 2.2 mm and 3.45 mm from the center of the ONH may be a superior measurement for distinguishing papilledema from crowded nerves,61 but this remains to be further validated. EDI OCT of the ONH allows for identification of drusen buried within the nerve substance,38, 65-67 which may help to rule out papilledema in patients in which there is otherwise a low clinical suspicion (Figure 6). However, OCT imaging of the ONH in pediatric patients following treatment of clinically definite papilledema found evidence of buried drusen in 50% of subjects,57 thus, the identification of optic disc drusen cannot by itself be taken as definitive proof of pseudopapilledema. Whether the angulation of Bruch’s membrane at the ONH54 is a more reliable indicator of the presence of elevated ICP in children remains to be determined.
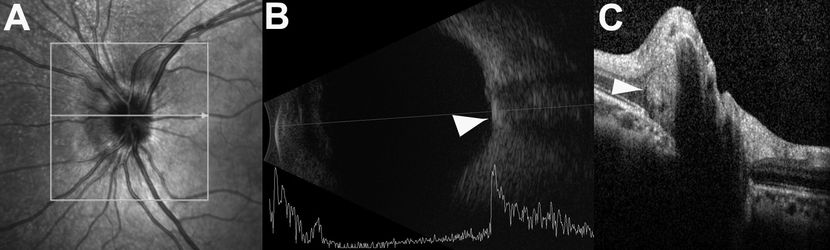
Figure 6. Pseudopapilledema in a child with buried ONH drusen. (A) Scanning laser ophthalmoscopy image of the left eye of a patient with pseudopapilledema. The ONH appears elevated with blurred margins, and it is easily mistaken for optic disc edema because the drusen are buried. (B) Optic disc drusen identified on B-scan ultrasonography as a hyperreflective area overlying the optic nerve (arrowhead). (C) Buried optic disc drusen easily identifiable on OCT as a rounded hyperreflectivity (arrowhead) within the ONH substance.
OCT has additional applications in evaluating patients with optic disc drusen, aside from a potential role in distinguishing it from papilledema. Decreased RNFL thickness seen in the setting of optic disc drusen is suggestive of partial optic atrophy and the presence of a visual field defect in children.68 Furthermore, optic disc drusen may result in the development of peripapillary choroidal neovascularization that may be identified on OCT by the appearance of exudative change adjacent to the drusen.69 This complication of optic disc drusen may be treated with anti-vascular endothelial growth factor (VEGF) injections and the response monitored by serial OCT imaging.70
Optic neuritis
ONH swelling in children can be due to a variety of other causes, including inflammation, malignant infiltration, mechanical compression by a mass lesion, and ischemia. Given its higher propensity for bilateral involvement of the optic nerves in children than in adults,71 optic neuritis is important to include in the differential diagnosis for children being evaluated for possible papilledema, especially in cases with severe vision loss. This inflammatory disease results in demyelination of the optic nerve, often with permanent residual visual dysfunction of varying severity due to a neurodegenerative component. Optic neuritis may occur in isolation or as a feature of more widespread disease also affecting the CNS, such as multiple sclerosis (MS) or neuromyelitis optica (NMO). Longitudinal monitoring is important to detect signs of recurrence and progressive visual decline in these conditions.
As in other diseases with ONH swelling, optic neuritis with anterior involvement will demonstrate thickening of the peripapillary RNFL on OCT during the acute phase (Figure 7);72 however, reports of RNFL thickening in cases of retrobulbar optic neuritis without clinically apparent ONH edema suggest that OCT may provide high sensitivity for detecting the disease in clinic.73 Optic atrophy typically begins to develop 4-6 weeks after the acute onset of symptoms, and this manifests on OCT as RNFL thinning correlating with final visual outcome.17,72,74,75 The GCL-IPL complex (representing the non-axonal cellular compartments of the RGCs) may develop thinning on macular OCT scans while RNFL edema has not yet resolved, thus serving as an early indicator of RGC dropout and possible permanent visual dysfunction.76,77 Furthermore, OCT imaging has revealed cystic lesions in the inner nuclear layer of patients with optic atrophy secondary to optic neuritis, believed to be either a manifestation of retrograde trans-synaptic neurodegeneration of second-order retinal neurons78,79 or a schisis-like phenomenon resulting from persistent vitreous traction on Müller cell endplates in areas of RGC dropout.80 Interestingly, MS patients without a history of optic neuritis have also been reported to have thinner RNFL measurements than healthy controls, a finding that correlates with the burden of whole brain atrophy noted on neuro-imaging of these patients.81, 82 Whether this is a manifestation of a primary neurodegenerative component of MS or of retrograde trans-synaptic degeneration from pathology of the posterior portions of the visual pathway is unclear, but the finding suggests the potential utility of serial OCTs as a means of measuring disease activity of MS, a much less expensive strategy than frequent magnetic resonance imaging (MRI). In fact, RNFL thickness is currently being used as one of the biomarkers for treatment efficacy in clinical trials of MS drugs.19
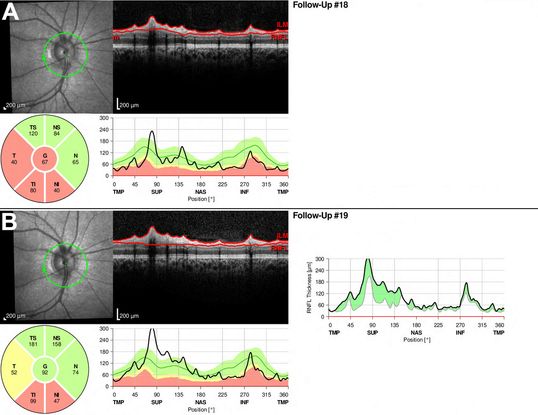
Figure 7. Recurrent optic neuritis demonstrated by OCT. (A) RNFL OCT of the right eye of a child with a prior history of optic neuritis. Note the abnormal thinning of the inferior and temporal quadrants of the peripapillary RNFL, consistent with optic atrophy. (B) When the patient was next seen in clinic after complaining of worsening vision, RNFL OCT revealed significant new thickening of the RNFL, predominantly in the superior and nasal quadrants which had not yet atrophied. The shaded area in the composite graph to the right demonstrates the interval change between scans. The OCT and clinical findings were consistent with recurrent optic neuritis.
While there have been few studies in the pediatric population, RNFL and total retinal thinning have similarly been demonstrated in children with optic neuritis and MS.83-85 Total macular volume on OCT was reported to be further decreased in patients with juvenile-onset MS compared to duration-matched adult-onset MS patients, suggesting that the pediatric retina may be particularly susceptible to atrophy in this disease. A shortcoming of the study was the lack of correlation with visual function.86 The clinical utility of serial OCT imaging in pediatric optic neuritis and/or MS patients awaits definitive study and will likely benefit from the incorporation of reliable age-based normative values into OCT software.
Neuroretinitis
The potential benefit of OCT imaging in neuroretinitis merits brief mention. This inflammatory disease of the optic disc vasculature features optic disc swelling as well as exudation into the macula, resulting in a classic macular star appearance on funduscopic examination. The etiology is typically infectious (eg, cat-scratch disease from Bartonella henselae), but may also be idiopathic. In addition to RNFL thickening due to ONH edema, OCT imaging in neuroretinitis also demonstrates foveal contour changes, retinal thickening, subretinal fluid and intraretinal hyperreflective exudates.87 In particular, the presence of focal intraretinal hyperreflectivities should prompt a workup for neuroretinitis even if a macular star is not observed clinically, as this finding would be highly atypical in optic neuritis or papilledema.
Compressive Optic Neuropathies and Intrinsic Tumors of the Visual Pathway
OCT studies in adults have demonstrated that pre-surgical RNFL and GCL-IPL measurements correlate well with postoperative visual function in patients with neoplasms compressing the anterior visual pathway.21,88,89 Furthermore, lesions compressing the posterior visual pathway may manifest as homonymous hemimacular thinning on OCT (Figure 8).90 In children, there are a number of neoplasms that may compress the optic chiasm or optic nerves, including craniopharyngioma, pituitary adenoma, and germ cell tumor, but the greatest utility for OCT may be in the management of patients with optic pathway gliomas (OPGs).91,92 Most commonly seen in young children with neurofibromatosis type I, OPGs are usually low-grade and indolent lesions. Clinical observation is the most common initial approach, with documentation of decreasing visual function prompting the clinician to pursue treatment options such as a challenging surgical resection, radiation, or chemotherapy.93 Because most patients are younger than 5 years old at the time of diagnosis, imaging-based proxies for the threat to visual function may be preferable to unreliable tests of visual acuity and visual field testing in the clinic. Progressive RNFL or GCL-IPL thinning may better correlate with declining visual function than do poorly predictive changes in lesion size on MRI,41, 94-96 particularly in light of reports that OCT measurements remain stable in patients without progressive visual dysfunction.97 Definitive trials will be required before OCT changes can be established as an appropriate trigger for initiating treatment in patients with OPGs, but this may prove to be one of the more valuable applications of OCT technology in pediatric ophthalmology.
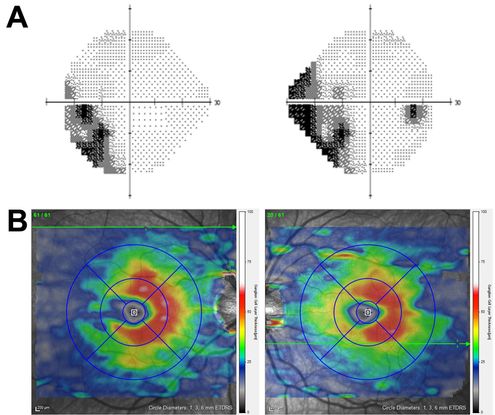
Figure 8. OCT correlate of homonymous visual field loss. (A) Humphrey visual field testing of the left and right eyes reveals a partial left homonymous visual field deficit in a child with a right occipital lobe lesion. (B) Macular map OCT (segmented for GCL-IPL thickness) demonstrates thinning nasal to the left fovea and temporal to the right fovea, which matches the visual field defect and is consistent with retrograde trans-synaptic degeneration of RGCs due to the CNS lesion.
Congenital optic disc anomalies
With improvement in the resolution and depth of OCT imaging, interest has grown in using the ONH map OCT protocol to evaluate the physical structure of the ONH in congenital optic disc anomalies. Two reports have shown that the ONH map corresponds well with the funduscopic appearance of morning glory disc anomaly, demonstrating an enlarged and highly excavated disc with minimal rim and a central glial tuft; additionally, there was reported peripapillary RNFL thickening temporally and nasally and macular thinning when compared to the uninvolved fellow eye.98,99 Swept-source OCT was recently used to evaluate a series of patients with optic pits and optic disc colobomas, demonstrating defects in the underlying lamina cribrosa with herniated retinal tissue separating the discs’ deep cups from the underlying subarachnoid space by merely 100 microns.36 Such insights may contribute to our mechanistic understanding of the development of schisis-like change and subretinal fluid in the peripapillary retina. Finally, an observational study of patients with optic nerve hypoplasia used ONH mapping to demonstrate smaller optic discs and cups in affected eyes and reported a tendency toward a hypoplastic foveal depression on macular OCT.100 It remains to be seen whether OCT will prove useful in predicting ultimate visual function in pediatric patients with these congenital disorders.
Conclusion
OCT imaging is not yet the standard of care in managing pediatric optic neuropathies, but its application in a wide variety of disease entities is under active investigation. Significant effort is now being directed to characterizing longitudinal changes in various OCT parameters and determining how quantitative and qualitative OCT findings may correlate with visual prognosis. Furthermore, the utility of OCT in pediatric ophthalmology will undoubtedly grow as age- and race-specific normative databases are created and integrated into OCT software. Given the rapid rate of technological improvement and the recent explosion in investigational applications, it seems inevitable that in the coming years there will be an OCT-driven revolution in the diagnosis and management of optic neuropathies, and that this technology will become a fixture in the pediatric ophthalmology clinic.
References
- Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181.
- Fercher AF. Optical coherence tomography - development, principles, applications. Z Med Phys. 2010;20(4):251-276.
- Simpson T, Fonn D. Optical coherence tomography of the anterior segment. Ocul Surf. 2008;6(3):117-127.
- Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98 Suppl 2:ii15-9.
- Onal S, Tugal-Tutkun I, Neri P, P Herbort C. Optical coherence tomography imaging in uveitis. Int Ophthalmol. 2014;34(2):401-435.
- Geitzenauer W, Hitzenberger CK, Schmidt-Erfurth UM. Retinal optical coherence tomography: past, present and future perspectives. Br J Ophthalmol. 2011;95(2):171-177.
- Nassif N, Cense B, Park B, et al. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004;12(3):367-376.
- Drexler W, Liu M, Kumar A, Kamali T, Unterhuber A, Leitgeb RA. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt. 2014;19(7):071412.
- Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496-500.
- Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013;58(5):387-429.
- Medeiros FA, Zangwill LM, Bowd C, Vessani RM, Susanna R, Jr., Weinreb RN. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139(1):44-55.
- Mrugacz M, Bakunowicz-Lazarczyk A. Optical coherence tomography measurement of the retinal nerve fiber layer in normal and juvenile glaucomatous eyes. Ophthalmologica. 2005;219(2):80-85.
- Ojima T, Tanabe T, Hangai M, Yu S, Morishita S, Yoshimura N. Measurement of retinal nerve fiber layer thickness and macular volume for glaucoma detection using optical coherence tomography. Jpn J Ophthalmol. 2007;51(3):197-203.
- Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47(5):2006-2010.
- Wessel JM, Horn FK, Tornow RP, et al. Longitudinal analysis of progression in glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(5):3613-3620.
- Klistorner A, Arvind H, Garrick R, Graham SL, Paine M, Yiannikas C. Interrelationship of optical coherence tomography and multifocal visual-evoked potentials after optic neuritis. Invest Ophthalmol Vis Sci. 2010;51(5):2770-2777.
- Noval S, Contreras I, Rebolleda G, Muñoz-Negrete FJ. Optical coherence tomography versus automated perimetry for follow-up of optic neuritis. Acta Ophthalmol Scand. 2006;84(6):790-794.
- Noval S, Contreras I, Rebolleda G, Muñoz-Negrete FJ. Optical coherence tomography in optic neuritis. Ophthalmology. 2007;114(1):200.
- Sergott RC. Optical coherence tomography: measuring in-vivo axonal survival and neuroprotection in multiple sclerosis and optic neuritis. Curr Opin Ophthalmol. 2005;16(6):346-350.
- Watson GM, Keltner JL, Chin EK, Harvey D, Nguyen A, Park SS. Comparison of retinal nerve fiber layer and central macular thickness measurements among five different optical coherence tomography instruments in patients with multiple sclerosis and optic neuritis. J Neuroophthalmol. 2011;31(2):110-116.
- Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci. 2008;49(5):1879-1885.
- Danesh-Meyer HV, Yap J, Frampton C, Savino PJ. Differentiation of compressive from glaucomatous optic neuropathy with spectral-domain optical coherence tomography. Ophthalmology. 2014;121(8):1516-1523.
- Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117(3):623-627.
- Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology. 2005;112(1):120-126.
- Ito Y, Nakamura M, Yamakoshi T, Lin J, Yatsuya H, Terasaki H. Reduction of inner retinal thickness in patients with autosomal dominant optic atrophy associated with OPA1 mutations. Invest Ophthalmol Vis Sci. 2007;48(9):4079-4086.
- Jimenez B, Ascaso FJ, Cristobal JA, López del Val J. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord. 2014;29(1):68-74.
- Kim YW, Kim SJ, Yu YS. Spectral-domain optical coherence tomography analysis in deprivational amblyopia: a pilot study with unilateral pediatric cataract patients. Graefes Arch Clin Exp Ophthalmol. 2013;251(12):2811-2819.
- Yanni SE, Wang J, Cheng CS, et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol. 2013;155(2):354-360 e1.
- Barrio-Barrio J, Noval S, Galdos M, et al. Multicenter Spanish study of spectral-domain optical coherence tomography in normal children. Acta Ophthalmol. 2013;91(1):e56-63.
- El-Dairi M, Holgado S, Asrani S, Freedman SF. Optical coherence tomography (OCT) measurements in black and white children with large cup-to-disc ratios. Exp Eye Res. 2011;93(3):299-307.
- El-Dairi MA, Asrani SG, Enyedi LB, Freedman SF. Optical coherence tomography in the eyes of normal children. Arch Ophthalmol. 2009;127(1):50-58.
- Leung MM, Huang RY, Lam AK. Retinal nerve fiber layer thickness in normal Hong Kong Chinese children measured with optical coherence tomography. J Glaucoma. 2010;19(2):95-99.
- Rao A, Sahoo B, Kumar M, Varshney G, Kumar R. Retinal nerve fiber layer thickness in children <18 years by spectral-domain optical coherence tomography. Semin Ophthalmol. 2013;28(2):97-102.
- Marsh BC, Cantor LB, WuDunn D, et al. Optic nerve head (ONH) topographic analysis by stratus OCT in normal subjects: correlation to disc size, age, and ethnicity. J Glaucoma. 2010;19(5):310-318.
- Ouyang Y, Heussen FM, Keane PA, Pappuru RK, Sadda SR, Walsh AC. Juxtapapillary pigment epithelium detachment observed in asymptomatic participants using optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(2):1144-1149.
- Ohno-Matsui K, Hirakata A, Inoue M, Akiba M, Ishibashi T. Evaluation of congenital optic disc pits and optic disc colobomas by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(12):7769-7778.
- Kulkarni KM, Pasol J, Rosa PR, Lam BL. Differentiating mild papilledema and buried optic nerve head drusen using spectral domain optical coherence tomography. Ophthalmology. 2014;121(4):959-963.
- Silverman AL, Tatham AJ, Medeiros FA, Weinreb RN. Assessment of optic nerve head drusen using enhanced depth imaging and swept source optical coherence tomography. J Neuroophthalmol. 2014;34(2):198-205.
- Langenegger SJ, Funk J, Toteberg-Harms M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Invest Ophthalmol Vis Sci. 2011;52(6):3338-3344.
- Rajjoub RD, Trimboli-Heidler C, Packer RJ, Avery RA. Reproducibility of retinal nerve fiber layer thickness measures using eye tracking in children with nonglaucomatous optic neuropathy. Am J Ophthalmol. 2015;159(1):71-77 e1.
- Avery RA, Cnaan A, Schuman JS, et al. Reproducibility of circumpapillary retinal nerve fiber layer measurements using handheld optical coherence tomography in sedated children. Am J Ophthalmol. 2014;158(4):780-787 e1.
- Avery RA, Hwang EI, Ishikawa H, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol. 2014;132(3):265-271.
- Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51(5):2678-2685.
- El-Dairi MA, Holgado S, Asrani SG, Enyedi LB, Freedman SF. Correlation between optical coherence tomography and glaucomatous optic nerve head damage in children. Br J Ophthalmol. 2009;93(10):1325-1330.
- Hess DB, Asrani SG, Bhide MG, Enyedi LB, Stinnett SS, Freedman SF. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol. 2005;139(3):509-517.
- Ghasia FF, Freedman SF, Rajani A, Holgado S, Asrani S, El-Dairi M. Optical coherence tomography in paediatric glaucoma: time domain versus spectral domain. Br J Ophthalmol. 2013;97(7):837-842.
- Prakalapakorn SG, Freedman SF, Lokhnygina Y, et al. Longitudinal reproducibility of optical coherence tomography measurements in children. J AAPOS. 2012;16(6):523-528.
- Ely AL, El-Dairi MA, Freedman SF. Cupping reversal in pediatric glaucoma--evaluation of the retinal nerve fiber layer and visual field. Am J Ophthalmol. 2014;158(5):905-915.
- Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87(7):899-901.
- Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. III. A pathologic study of experimental papilledema. Arch Ophthalmol. 1977;95(8):1448-1457.
- Wang JK, Kardon RH, Kupersmith MJ, Garvin MK. Automated quantification of volumetric optic disc swelling in papilledema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):4069-4075.
- Savini G, Bellusci C, Carbonelli M, et al. Detection and quantification of retinal nerve fiber layer thickness in optic disc edema using stratus OCT. Arch Ophthalmol. 2006;124(8):1111-1117.
- Scott CJ, Kardon RH, Lee AG, Frisén L, Wall M. Diagnosis and grading of papilledema in patients with raised intracranial pressure using optical coherence tomography vs clinical expert assessment using a clinical staging scale. Arch Ophthalmol. 2010;128(6):705-711.
- Kupersmith MJ, Sibony P, Mandel G, Durbin M, Kardon RH. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52(9):6558-6564.
- Rebolleda G, Muñoz-Negrete FJ. Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(11):5197-5200.
- Sibony P, Kupersmith MJ, Honkanen R, Rohlf FJ, Torab-Parhiz A. Effects of lowering cerebrospinal fluid pressure on the shape of the peripapillary retina in intracranial hypertension. Invest Ophthalmol Vis Sci. 2014;55(12):8223-8231.
- Gospe SM 3rd, Bhatti MT, El-Dairi MA. Anatomic and visual function outcomes in paediatric idiopathic intracranial hypertension. Br J Ophthalmol. 2016;100(4):505-509.
- Chen JJ, Thurtell MJ, Longmuir RA, et al. Causes and Prognosis of Visual Acuity Loss at the Time of Initial Presentation in Idiopathic Intracranial Hypertension. Invest Ophthalmol Vis Sci. 2015;56(6):3850-3859.
- Goldhagen BE, Bhatti MT, Srinivasan PP, Chiu SJ, Farsiu S, El-Dairi MA. Retinal atrophy in eyes with resolved papilledema detected by optical coherence tomography. J Neuroophthalmol. 2015;35(2):122-126.
- Heidary G, Rizzo JF 3rd. Use of optical coherence tomography to evaluate papilledema and pseudopapilledema. Semin Ophthalmol. 2010;25(5-6):198-205.
- Fard MA, Fakhree S, Abdi P, Hassanpoor N, Subramanian PS. Quantification of peripapillary total retinal volume in pseudopapilledema and mild papilledema using spectral-domain optical coherence tomography. Am J Ophthalmol. 2014;158(1):136-143.
- Lee KM, Woo SJ, Hwang JM. Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography. Ophthalmology. 2011;118(5):971-977.
- Karam EZ, Hedges TR. Optical coherence tomography of the retinal nerve fibre layer in mild papilloedema and pseudopapilloedema. Br J Ophthalmol. 2005;89(3):294-298.
- Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(1):45-49.
- Sato T, Mrejen S, Spaide RF. Multimodal imaging of optic disc drusen. Am J Ophthalmol. 2013;156(2):275-282 e1.
- Yi K, Mujat M, Sun W, et al. Imaging of optic nerve head drusen: improvements with spectral domain optical coherence tomography. J Glaucoma. 2009;18(5):373-378.
- Merchant KY, Su D, Park SC, et al. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology. 2013;120(7):1409-1414.
- Noval S, Visa J, Contreras I. Visual field defects due to optic disk drusen in children. Graefes Arch Clin Exp Ophthalmol. 2013;251(10):2445-2450.
- Duncan JE, Freedman SF, El-Dairi MA. The incidence of neovascular membranes and visual field defects from optic nerve head drusen in children. J AAPOS. 2016;20(1):44-48.
- Knape RM, Zavaleta EM, Clark CL 3rd, Khuddus N, Peden MC. Intravitreal bevacizumab treatment of bilateral peripapillary choroidal neovascularization from optic nerve head drusen. J AAPOS. 2011;15(1):87-90.
- Kennedy C, Carroll FD. Optic neuritis in children. Arch Ophthalmol. 1960;63:747-755.
- Kupersmith MJ, Mandel G, Anderson S, Meltzer DE, Kardon R. Baseline, one and three month changes in the peripapillary retinal nerve fiber layer in acute optic neuritis: relation to baseline vision and MRI. J Neurol Sci. 2011;308(1-2):117-123.
- Pro MJ, Pons ME, Liebmann JM, et al. Imaging of the optic disc and retinal nerve fiber layer in acute optic neuritis. J Neurol Sci. 2006;250(1-2):114-119.
- Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963-969.
- Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58(3):383-391.
- Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for optic neuritis. Mult Scler. 2016;22(5):641-648.
- Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77(3):517-528.
- Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One. 2013;8(8):e71145.
- Lujan BJ, Horton JC. Microcysts in the inner nuclear layer from optic atrophy are caused by retrograde trans-synaptic degeneration combined with vitreous traction on the retinal surface. Brain. 2013;136(Pt 11):e260.
- Jiramongkolchai K, Bhatti MT, Proia A, Freedman SF, El-Dairi MA. Formation of Macular Inner Nuclear Layer Cysts in Optic Atrophy. Invest Ophthalmol Vis Sci. 2016;57(3):989-991.
- Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749-760.
- Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69(16):1603-1609.
- Waldman AT, Hiremath G, Avery RA, et al. Monocular and binocular low-contrast visual acuity and optical coherence tomography in pediatric multiple sclerosis. Mult Scler Relat Disord. 2013;3(3):326-334.
- Yeh EA, Weinstock-Guttman B, Lincoff N, et al. Retinal nerve fiber thickness in inflammatory demyelinating diseases of childhood onset. Mult Scler. 2009;15(7):802-810.
- Yilmaz U, Gucuyener K, Erin DM, et al. Reduced retinal nerve fiber layer thickness and macular volume in pediatric multiple sclerosis. J Child Neurol. 2012;27(12):1517-1523.
- Huhn K, Lammer R, Oberwahrenbrock T, et al. Optical coherence tomography in patients with a history of juvenile multiple sclerosis reveals early retinal damage. Eur J Neurol. 2015;22(1):86-92.
- Habot-Wilner Z, Zur D, Goldstein M, et al. Macular findings on optical coherence tomography in cat-scratch disease neuroretinitis. Eye (Lond). 2011;25(8):1064-1068.
- Loo JL, Tian J, Miller NR, Subramanian PS. Use of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomas. Br J Ophthalmol. 2013;97(11):1455-1458.
- Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci. 2011;52(11):8527-8533.
- Oh J, Sotirchos ES, Saidha S, et al. In vivo demonstration of homonymous hemimacular loss of retinal ganglion cells due to a thalamic lesion using optical coherence tomography. JAMA Neurol. 2013;70(3):410-411.
- Avery RA, Rajjoub RD, Trimboli-Heidler C, Waldman AT. Applications of optical coherence tomography in pediatric clinical neuroscience. Neuropediatrics. 2015;46(2):88-97.
- Bialer OY, Goldenberg-Cohen N, Toledano H, Snir M, Michowiz S. Retinal NFL thinning on OCT correlates with visual field loss in pediatric craniopharyngioma. Can J Ophthalmol. 2013;48(6):494-499.
- Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol. 2011;31(3):269-278.
- Avery RA, Bouffet E, Packer RJ, Reginald A. Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54(2):1034-1038.
- Chang L, El-Dairi MA, Frempong TA, et al. Optical coherence tomography in the evaluation of neurofibromatosis type-1 subjects with optic pathway gliomas. J AAPOS. 2010;14(6):511-517.
- Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9(4):430-437.
- Avery RA, Cnaan A, Schuman JS, et al. Intra- and inter-visit reproducibility of ganglion cell-inner plexiform layer measurements using handheld optical coherence tomography in children with optic pathway gliomas. Am J Ophthalmol. 2014;158(5):916-923.
- Srinivasan G, Venkatesh P, Garg S. Optical coherence tomographic characteristics in morning glory disc anomaly. Can J Ophthalmol. 2007;42(2):307-309.
- Wu YK, Wu TE, Peng PH, Cheng CK. Quantitative optical coherence tomography findings in a 4-year-old boy with typical morning glory disk anomaly. J AAPOS. 2008;12(6):621-622.
- Pilat A, Sibley D, McLean RJ, Proudlock FA, Gottlob I. High-Resolution Imaging of the Optic Nerve and Retina in Optic Nerve Hypoplasia. Ophthalmology. 2015;122(7):1330-1339.