Establishing the Diagnosis
Embryology and anatomy
The lacrimal drainage structures begin to form during the fifth week of gestation as a crease between the frontonasal and maxillary processes, the nasolacrimal groove. A solid cord of ectodermal tissues separates from the surface and enters this groove. This tissue canalizes and forms the lacrimal sac and nasolacrimal duct. The lacrimal canaliculi form by a similar process. Canalization begins around the eighth week of gestation and continues until birth. Canalization occurs along the entire system at the same time. The opening between the nasolacrimal duct and the nares at the distal valve of Hasner is often not patent at birth.
Tears are produced in the lacrimal gland. They cross the eye, enter the upper and lower eyelid punctum, and travel through the canaliculi to the lacrimal sac and then into the nares via the nasolacrimal duct. Contraction of the orbicularis muscles creates a pumping action that facilitates the flow of tears through the lacrimal system.
Clinical symptoms of congenital nasolacrimal duct obstruction
Approximately 5% of infants have some symptoms of NLDO (nasolacrimal duct obstruction). It is usually caused by persistence of a membrane at the distal valve of Hasner. The primary symptoms are epiphora, which result from backflow of tears due to blockage of the duct, and periocular crusting and discharge due to infection of the lacrimal system (Figure 1). This dacryocystitis in infants with NLDO is typically low grade, and the organisms are usually normal flora. The infection results from stagnation of bacteria in the warm, moist environment of the lacrimal sac. Pressure over the lacrimal sac often produces retrograde reflux of mucopurulent material through the lacrimal puncta.
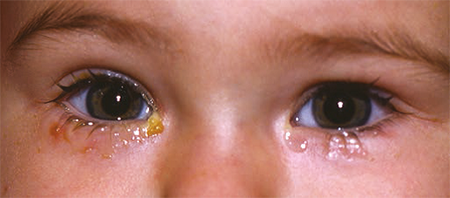
Figure 1. Bilateral nasolacrimal duct obstruction with epiphora and periocular crusting.
Most children with NLDO have both epiphora and periocular discharge, but some will have only one or the other. If epiphora is the only symptom, the possibility of canalicular atresia should be considered (see below), and it is imperative to rule out congenital glaucoma.
Of note, typical NLDO does not usually cause much discomfort to patients. Affected infants often act normally despite the presence of significant overflow tears and mucopurulent discharge. If infants have photophobia or other signs of irritation, they should be checked carefully for signs of glaucoma or corneal disease (see below). The absence of corneal and conjunctival abnormalities is an important factor in establishing the diagnosis of NLDO.
In children with more severe NLDO, erythema and breakdown of the periocular skin may occur due to near-constant exposure to fluid. Enlargement and abscess formation may occur in the lacrimal sac in infants with dacryocystoceles (discussed below).
Diagnosis/Differential Diagnosis
NLDO is by far the most common cause of epiphora and periocular discharge in infants. The presence of enlarged tear lakes and periocular discharge helps confirm the diagnosis. The vast majority of infants with these findings will have NLDO.
The most important entity in the differential diagnosis of NLDO is infantile glaucoma. NLDO may be confused with glaucoma by primary care physicians due to the presence of epiphora. Ophthalmologists easily establish the diagnosis by evaluating for associated findings seen in glaucoma: corneal enlargement, Haab striae, enlarged globe, increased cup:disc ratio, and elevated intraocular pressure. When educating primary care physicians about NLDO, it is important to stress the need for ophthalmic evaluation of infants who have photophobia and other signs of ocular irritation (eg, excessive eye rubbing) to rule out this possibility.
Any disorder that causes corneal irritation in infants may also be confused with NLDO. Among these disorders are epiblepharon (which may cause irritation due to in-turned eyelashes), primary corneal disorders, and corneal infection. These entities can be identified by the presence of associated eyelid or corneal abnormalities. Conjunctivitis may also cause epiphora and ocular discharge but is easily differentiated from NLDO by conjunctival changes such as injection, chemosis, and follicles.
Treatment
Non-surgical treatment
The majority of infants with NLDO spontaneously improve during the first several months of life. Therefore, most primary care physicians appropriately treat these patients initially with conservative measures. If infants have only mild symptoms, there may be no need for treatment. If significant epiphora or discharge is present, digital massage of the lacrimal sac is commonly recommended. The goal of massage is to force fluid through the distal NLD and cause the obstruction to open. If massage is used, it is important to demonstrate proper technique, which requires direct digital pressure over the lacrimal sac. The presence of mucopurulent reflux through the puncta indicates that pressure is being applied appropriately. It is not necessary to stroke the finger in a downward motion over the lacrimal sac as commonly taught; compression of the lacrimal sac is the only requirement to proper massage.
Treatment with topical antibiotics is sometimes recommended if there is significant discharge. It is important that caregivers understand that the benefits of topical antibiotics are usually temporary, and symptoms often recur when they are discontinued.
Surgical treatment
Clinic vs. operating room (OR) probing
If children do not improve with time and conservative measures, surgical treatment is indicated. NLD probing has a fairly high success rate. There are two main approaches to surgery.
Some ophthalmologists perform in-office probing in awake infants, typically at an early age (6 months or younger). The benefits of this approach are earlier resolution of NLDO and the avoidance of general anesthesia. The downside is increased discomfort and the treatment of infants who would spontaneously improve with additional time.
Other ophthalmologists recommend waiting until infants are older and performing NLD probing in the operating room under anesthesia. The benefits of this approach are less discomfort, avoiding treatment of infants who spontaneously improve after the age at which in-office probing is performed, and the ability to perform additional procedures if other abnormalities are found while the child is anesthetized. The primary downside is the risk of general anesthesia.
Cost is also a factor when comparing these two approaches. The cost of performing the procedure in the operating room is higher, but this is partially offset by avoiding treatment of many infants, postponing surgery instead until they are older.1
Routine NLD Probing
The objective of NLD probing is to establish a connection between the NLD and the nares by removing the obstruction at the distal duct. Whether done in the office or the operating room, this is achieved by passing a probe through the punctum, along the canaliculus to the lacrimal sac, and down into the nares. The probe is first placed nearly perpendicular to the eyelid through the lacrimal punctum, then quickly turned to follow the course of the canaliculus parallel to the lid margin. Usually, bone is felt when the probe encounters the nasal wall on the medial side of the lacrimal sac. It may be helpful to apply tension to the lower eyelid at the lateral canthus to facilitate passage of the probe to the lacrimal sac. The probe is then turned approximately 90 degrees and passed into the distal duct, through the obstruction into the nares (Figure 2 ). In most infants a popping sensation is felt as the probe passes through the obstruction.
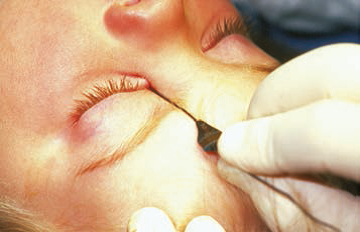
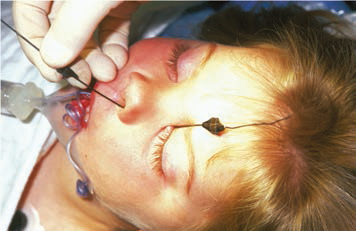
Figure 2. Probing for lacrimal obstruction. Left: The probe enters through the lower canaliculus. Right: A second probe enters the nares.
A variety of probes may be used for this procedure. Some ophthalmologists pass a single probe (particularly those who perform the procedure in the office), while others pass a sequence of larger probes to increase the size of the opening.2 The presence of metal-on-metal contact when a second probe is passed into the nares to palpate the probe in the NLD can confirm that the probe has been passed successfully, and may help confirm that a NLD cyst or other abnormality is not present. Most practitioners perform some sort of irrigation using a cannula after probing to ensure patency of the duct.
The inferior turbinate can be infractured to increase the space where the NLD exits into the nares. Some ophthalmologists perform this routinely. Others infracture the turbinate only if a particularly tight space is identified, or reserve it for treatment of patients who fail a first NLD probe.
The use of vasoconstrictors such as oxymetazoline can decrease bleeding during the procedure. They may be applied either directly via a soaked pledget or by nasal spray.
Some bleeding is common postoperatively, and can usually be controlled by digital pressure or the home use of topical vasoconstrictors. Many practitioners prescribe a short course of topical antibiotics with or without corticosteroids. Pain can usually be controlled with oral acetaminophen or ibuprofen, and most infants recover quickly following the procedure. The success rate of NLDO is approximately 80%.3
Unusual congenital lacrimal disorders
Punctal/canalicular atresia
Obstruction of the upper portion of the lacrimal system is an uncommon cause of lacrimal obstruction in infants. If both the upper and lower eyelids are involved, patients present with epiphora only, because bacteria do not have access to the lacrimal sac. Therefore, the presence of epiphora without periocular discharge should raise the possibility of this type of abnormality. However, because NLDO is very common and punctal or canalicular abnormalities are relatively rare, most infants who only have symptoms of epiphora will have typical NLDO.
In some patients, the lacrimal puncta are blocked by a membrane that covers the opening. Puncturing the membrane with a punctal dilator usually easily treats this. In other patients, the canaliculi themselves are not present. In these patients, no punctal dimple is present on the eyelid. (Figure 3 ) If both upper and lower canaliculi are absent, treatment with a Jones tube (conjunctivodacryocystorhinostomy) is necessary. This is usually deferred until an older age. If only one eyelid is affected, most patients present with symptoms of lacrimal infection in addition to epiphora, indicating that a distal obstruction is also present (because the bacteria must be able to access the lacrimal sac through the patent canaliculus and the distal duct must be blocked in order for infection to develop). In these patients, nasolacrimal duct probing (NLDP) performed through the normal eyelid may be curative.4
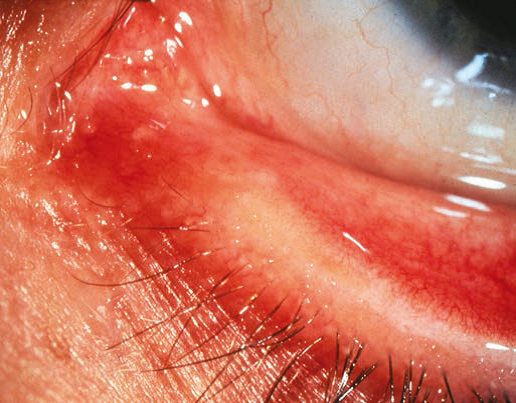
Figure 3. Atresia of the lacrimal puncta.
Neonatal dacryocystocele
Approximately 3% of infants with NLDO present in the neonatal period with a dacryocystocele. Dacryocystoceles result from distention of the lacrimal sac, creating a visible blue mass in the skin overlying the lacrimal sac (Figure 4 ). Almost all of these are associated with a cystic lesion in the nares, caused by distention of the membrane that covers the distal NLD (Figure 5).
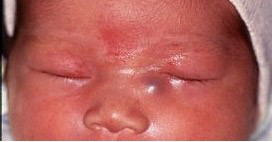
Figure 4. Dacryocystocele.
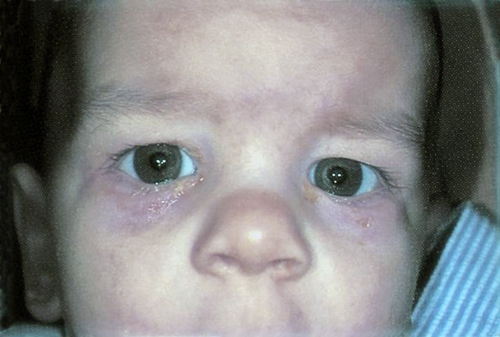
Figure 5. Nasolacrimal duct obstruction.
Dacryocystoceles are clinically important for two reasons. First, if the nasolacrimal duct cysts are large, they may cause respiratory problems in newborns. This can range from acute respiratory distress due to complete occlusion of the nares (which requires emergent endoscopy and cyst removal), to intermittent feeding difficulties (caused by NLD cysts that obstruct the airway and become symptomatic when the infant’s mouth is covered during feeding).
The second important clinical problem in these patients is the potential for acute lacrimal infection. Unlike the low-grade, chronic dacryocystitis found in typical NLDO, an acute lacrimal sac abscess often results from the infection associated with dacryocystoceles, with distention and erythema visible at the lacrimal sac. Because infants are relatively immunocompromised in the first few months of life, these infections have the potential to spread, causing serious problems such as orbital cellulitis, meningitis, or sepsis. Therefore, prompt treatment of these infected dacryocystoceles is indicated.
If the dacryocystocele is not infected and is not causing respiratory problems, initial conservative treatment often results in resolution of the lesion. Warm compresses and topical antibiotics are usually recommended. Digital pressure over the mass may induce resolution.
If the dacryocystocele becomes acutely infected, further measures are indicated. Systemic antibiotics should be used in such patients. Some practitioners perform NLD probing in these patients, which is effective in approximately 75% of them.5 NLD cysts are almost universally present in this disorder. Examination with either a nasal speculum or an endoscope may help identify these cysts. Lacrimal probing and endoscopic removal of the cyst is curative in > 95% of patients.5
Diffuse stenosis of distal duct (found during probing)
Most infants with NLDO have membranes that are easily opened when probes are passed during surgery. A mild popping sensation is felt when the probe penetrates the membrane. This is curative in the majority of patients.
Less commonly, diffuse stenosis of the distal duct is present (Figure 6). When the probe is passed, obstruction is palpable along the course of the distal duct, rather than just at its entry into the nares. The passage typically feels gritty or crunchy, as if the probe is being passed through sand or gravel. It is important to recognize this distinction, because patients with diffuse stenosis have a lower rate of success with NLD probing. Balloon catheter dilation (BCD) may improve the success rate.6
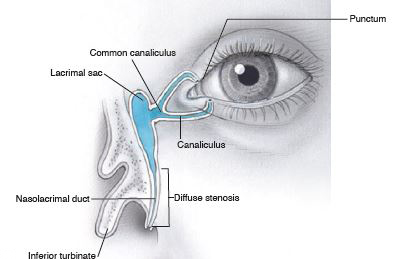
Figure 6. Stenosis of the distal duct.
If increased resistance is palpated during NLD probing, it is important to distinguish between diffuse stenosis and a false passage, as they produce similar sensations. Nasal examination with a headlight or endoscope can help make this distinction.
Older age at initial probing
Some earlier studies suggested that the success rate of NLD probing was lower in older children, whereas other studies found no difference.7 This discrepancy can be largely explained by the difference between typical membranous obstruction of the duct and diffuse stenosis. Patients with typical obstruction do well with surgery, regardless of age, while those with diffuse stenosis are less likely to improve.
Patients who require repeat surgery
The success rate of initial NLD probing ranges from approximately 75-90% in reported studies.1, 3 A variety of treatment options are available for children requiring repeat surgery. Some surgeons simply repeat probing as a first choice. Other options are discussed below.
Stents
Lacrimal stents are used to reduce the risk of recurrent stenosis of the NLD duct. They are usually reserved for patients who have persistent symptoms after initial NLD probing. Some surgeons have suggested that stents be placed in all patients during initial surgery.8 Studies suggest this does increase the success rate of surgery, but it necessitates the placement of probes in a majority of patients who would have done well without them. Given the increased complexity of surgery, potential stent complications, need for removal, and increased cost, most surgeons do not follow this practice.
A wide variety of stents are available. Bicanalicular stents are placed through the upper and lower puncta and are usually secured in the nares with a suture. A stent with an intraluminal suture is available that can be tied to itself. This makes removal of the stent easier, but increases the risk of stent displacement. Securing the stent with a suture to mucosa in the nares is commonly done, and these stents can usually be removed in the clinic. Securing with a bolster, such as a piece of retinal explant sponge, is the most secure way to fixate the stent, but may require anesthesia for removal. Monocanalicular stents can be placed through the upper or lower puncta (sometimes both). The primary advantage of monocanalicular stents is ease of removal, but they are more prone to dislodging.
Complications of stent placement include early displacement, elongation of the lacrimal puncta, and corneal abrasion. These problems may require early removal. Stents are usually left in place for 2-6 months.
Balloon catheter dilation
During balloon catheter dilation (BCD), a stent with a balloon at its distal end is passed into the distal nares, the balloon is inflated (typically two times in both the proximal and distal duct), then deflated and removed.9 A typical inflation is to 8-10 atm for 90 seconds then an additional inflation to 8-10 atm for 60 seconds at each location. The goal is to widen the distal duct and decrease obstruction. The primary advantage of BCD over stents is that no stent material is left in the lacrimal system and stent removal is not required. The primary disadvantage is the cost of the instruments and the increased operating time. BCD is particularly useful for patients with diffuse stenosis of the distal NLD.
Turbinate infracture
Infracture of the inferior turbinate, usually done with an instrument such as a periosteal elevator or a hemostat, is sometimes used to decrease the resistance of drainage in the distal nasolacrimal duct. It is particularly useful for patients who have a very tight space between the inferior turbinate and nasal wall. Some surgeons perform this routinely, while others reserve its use for patients in whom previous probing has failed.
Nasal endoscopy
Nasal endoscopy can be a useful adjunct to the management of patients with NLD, particularly in certain clinical situations. As discussed above, infants with congenital dacryocystocele almost always have associated cysts of the distal NLD. Removal of these cysts, which is most easily accomplished with the use of an endoscope, increases the success rate of surgery. Similar cystic lesions may also be seen in older infants with particularly severe symptoms of NLDO, and account for some patients with persistent symptoms after initial NLD probing. If found, distal NLD cysts can be removed with alligator forceps or other instruments via the nostril. Some ophthalmologists perform endoscopy themselves, while others do these procedures in conjunction with otolaryngologists.
Dacryocystorhinostomy
Dacryocystorhinostomy (DCR) involves creation of a new opening between the lacrimal sac and nasal cavity, usually at the level of the lacrimal sac itself. In children, this procedure is reserved for patients who have persistent symptoms despite undergoing the procedures described above. DCR can be performed via a skin incision, with exposure of the lacrimal sac, creation of an osteotomy through the nasal bone, formation of flaps between the lacrimal sac and nasal mucosa, and placement of lacrimal stents. Laser probes are an alternative, with creation of an ostium utilizing a laser placed through the canaliculus and adjacent to the nasal bone. Endoscopy is usually used during laser DCR, and stents are placed at the end of the procedure.
References
- A randomized trial comparing the cost-effectiveness of 2 approaches for treating unilateral nasolacrimal duct obstruction. Arch Ophthalmol. 2012; 130:1525-1533.
- Clark RA. Dilation probing as primary treatment for congenital nasolacrimal duct obstruction. J AAPOS. 2002; 6:364-367.
- Pediatric Eye Disease Investigator Group, Repka MX, Chandler DL, Beck RW, Crouch ER 3rd, Donahue S, Holmes JM, Lee K, Melia M, Quinn GE, Sala NA, Schloff S, Silbert DI, Wallace DK. Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 years. Ophthalmology. 2008; 115:577-584.
- Soliman M, Lueder GT. Initial management of congenital canalicular atresia. J AAPOS. 2015; 19:220-222.
- Lueder GT. The association of neonatal dacryocystoceles and infantile dacryocystitis with nasolacrimal duct cysts (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2012; 110:74-93.
- Lueder GT. Balloon catheter dilation for treatment of older children with nasolacrimal duct obstruction. Arch Ophthalmol. 2002;120:1685-1688.
- Paul TO, Shepherd R. Congenital nasolacrimal duct obstruction: Natural history and the timing of optimal intervention. J Pediatr Ophthalmol Strabismus. 1994; 31:362-367.
- Engel JM, Hichie-Schmidt C, Khammar A, Ostfeld BM, Vyas A, Ticho BH. Monocanalicular silastic intubation for the initial correction of congenital nasolacrimal duct obstruction. J AAPOS. 2007; 11:183-186.
- Becker BB, Berry FD, Koller H. Balloon catheter dilatation for treatment of congenital nasolacrimal duct obstruction. Am J Ophthalmol. 1996; 121:304.