This chapter was reviewed for currency and updated in April 2021 by Sapna Gangaputra, MD, MPH, and H. Nida Sen, MD, MHS.
Introduction
Childhood uveitis is a complex condition fraught with challenges in thorough examination, delays in diagnosis, and high ocular morbidity. It is less common than adult uveitis and accounts for 5%–10% of all uveitis seen in tertiary referral clinics.1 A claims-based database estimated the prevalence of pediatric noninfectious anterior uveitis at 22 per 100,000 persons (95% CI, 19.3-25.4).2 In a review of 9 large series, uveitis was seen slightly more frequently in girls than boys.3 Among all children with intraocular inflammation, anterior uveitis accounted for 30%–40% of all cases, posterior uveitis for 40%–50%, intermediate uveitis for 10%–20%, and panuveitis for 5%–10%.3 The most common cause of anterior uveitis in childhood is juvenile idiopathic arthritis (JIA). The most common causes of vision loss in pediatric anterior uveitis patients are cataract, band keratopathy, glaucoma, and cystoid macular edema (CME).3,4 Severe vision loss has been estimated to occur in 25%–30% of pediatric uveitis cases, making prompt diagnosis and rigorous treatment essential to preserve vision in children with uveitis.1–5
Etiology and Clinical Features
Juvenile Idiopathic Arthritis
JIA is the most common systemic association of pediatric uveitis.6,7 It is defined as inflammation of one or more joints, of unknown etiology, that begins before the age of 16 years and persists for at least 6 weeks.8 Uveitis secondary to JIA is an important cause of pediatric uveitis in North America, and has an estimated incidence of approximately 4.9 to 6.9 per 100,000 person-years and an estimated prevalence of 13 to 30 per 100,000 person-years.9
Several classification systems exist for juvenile arthritis, including the European League Against Rheumatism (EULAR),1,10 the American College of Rheumatology and the American Rheumatism Association (ACR/ARA),11 and the International League of Associations of Rheumatologists (ILAR) classifications.12 The most widely used in North America is the ACR/ARA system,1,8 which divides the chronic juvenile arthritides into 3 broad categories:
- Juvenile-onset spondyloarthropathies (JOSpAs)
- JIA/juvenile rheumatoid arthritis
- Other arthritides in childhood (sarcoidosis, etc.)
JOSpAs include juvenile ankylosing spondylitis, reactive arthritis, inflammatory bowel disease (IBD)–related arthritis — the 3 also collectively known as enthesitis-related arthritis — and psoriatic arthritis. JIA is further classified into 3 categories:
- Systemic (Still disease) Oligoarticular7
- Polyarticular
- Oligoarticular8
Systemic Onset
This subtype accounts for approximately 10% of all patients with JIA. It is usually seen in children younger than 5 years of age and occurs with equal frequency in boys and girls. It is characterized by prominent systemic manifestations of fever, rash, lymphadenopathy, hepatosplenomegaly, polyarthritis, pericarditis, and peritonitis.8,13 The fever is often present in the evening and is accompanied by a characteristic faint, erythematous, macular rash that is evanescent, salmon-colored, linear, or circular and is most commonly distributed over the trunk and proximal extremities. Cutaneous hypersensitivity to superficial trauma (the Koebner phenomenon) is often present. The arthritis in systemic JIA can affect any number of joints, is classically polyarticular and destructive, and can affect the hip, cervical spine, and temporomandibular joint. Macrophage activation syndrome, which can be a life-threatening manifestation, can develop in these patients.8,13 Ocular involvement is rare and fewer than 6% of patients develop uveitis.8,13
Polyarticular Onset
This subtype is characterized by involvement of more than 4 joints in the first 6 months of disease. It is more common in females and can be further subdivided into rheumatoid factor (RF)‑positive and RF‑negative polyarthritis. When RF is positive, the age of onset is typically older, between 9 and 12 years.7 Polyarticular disease resembles the characteristic symmetric presentation of adult rheumatoid arthritis. Rheumatoid nodules on the extensor surfaces of the elbows and over the Achilles tendons are associated with a more severe course.7 This type accounts for less than 10% of all cases of JIA and the patients typically do not develop uveitis.1 In RF-negative polyarthritis, which accounts for 20%–30% of all JIA, large and small joints can be involved, either symmetrically or asymmetrically. Uveitis occurs in 5%–10% of these patients.1,7,12
Pauciarticular Onset
This subtype accounts for 50%–60% of all JIA and is characterized by involvement of 4 or fewer joints within the first 6 months of presentation. Occasionally patients have no joint symptoms. Age of onset is usually less than 6 years, and it is more common among females. Joints involved are frequently the knees, ankles, and fingers.8 Approximately 30%–50% of children with pauciarticular JIA develop uveitis.1,13 The antinuclear antibody (ANA) is commonly positive and confers increased risk of ocular involvement.8,13
The average age of onset of uveitis in these patients is 6 years and it typically develops within 5–7 years of the onset of joint disease. The eyes typically appear white and uninflamed on gross examination, so slit-lamp examination for evaluation of intraocular inflammation is key.13–15 The uveitis is generally asymptomatic; therefore, patients with JIA require screening slit-lamp examination for diagnosis. JIA-associated uveitis is chronic, typically nongranulomatous, with predominantly bilateral iridocyclitis. Keratic precipitates (KP) are usually small and nongranulomatous, although larger granulomatous KP can be seen as well.16 Anterior chamber cells and flare are present and there can be cells found in the anterior vitreous from inflammation of the ciliary body.
Long-standing inflammation can lead to development of band keratopathy, posterior synechiae, pupillary membranes that can become vascularized, cataract, and glaucoma (Figure 1). Macular edema, chronic hypotony, and phthisis can develop without adequate treatment.14–16 There is usually little or no correlation between the joint activity and eye findings; therefore, joint disease cannot be used as an indicator of ocular disease. Because joint disease can be minimal or absent when uveitis is diagnosed, all children in whom JIA is suspected should be evaluated by a pediatric rheumatologist.1,8,16
JIA is a multifactorial and polygenetic disease.17 The complex pathogenesis of JIA-associated uveitis is poorly understood. Much of the pathophysiological data are derived from studies in experimental animal models. These studies have focused on the principal cells of the adaptive immune system, in particular different subsets of regulatory and effector T‑cells, as well as on antigen-presenting cells or dendritic cells. The available data point to the development of aberrant immune responses involving both the innate and adaptive immune responses.17,18
Ocular conditions to be considered in the differential diagnosis include ocular sarcoidosis and Blau syndrome, which can also present with joint and eye involvement. These 2 conditions tend to present with granulomatous ocular inflammation and can have posterior segment involvement, which is atypical in JIA-related uveitis. Children with seronegative spondyloarthropathies such as ankylosing spondylitis can also present with anterior uveitis; however, this inflammation tends to present acutely, with pain, redness, and photophobia, and tends to involve 1 eye at a time.
Due to the asymptomatic nature of the disease, its long-term prognosis depends not only on the extent of damage at the time of diagnosis, but also on the timely institution of appropriate and aggressive therapy to prevent complications.19–21 Ocular screening guidelines for children with JIA are outlined in Table 1.22 Children who are diagnosed with uveitis secondary to JIA need regular ocular examinations when disease control has been achieved, at least every 2–3 months, sometimes more frequently, depending on the severity of the disease.
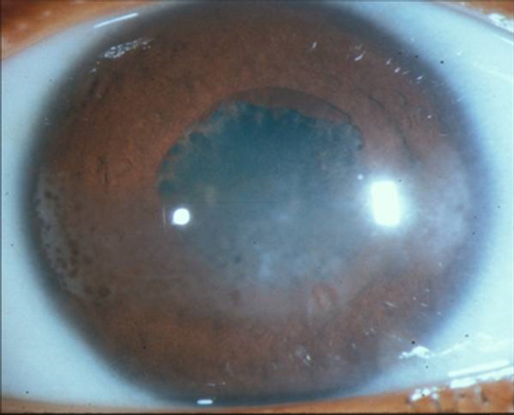
Figure 1. Child with juvenile idiopathic arthritis–associated uveitis. Note the band keratopathy with "Swiss cheese" pattern most prominent nasally and temporally. There are posterior synechiae between the pupillary margin and the anterior lens capsule and early pupillary membranes at the leading edge of the synechiae.
Table 1. Recommended screening schedule for JIA/JIA patients without known iridocyclitis.
|
JIA Subtype
|
Age of Onset
|
|
< 7 Years
|
> 7 Years
|
|
Pauciarticular
ANA (+)
ANA (-)
|
Every 3–4 months
Every 6 months
|
Every 6 months
Every 6 months
|
|
Polyarticular
ANA (+)
ANA (-)
|
Every 3–4 months
Every 6 months
|
Every 6 months
Every 6 months
|
|
Systemic
|
Every 12 months
|
Every 12 months
|
Adapted from American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology: Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 1993;92(2):295–296. Updated recommendations from ACR 2019.23
Juvenile-Onset Spondyloarthropathies (JOSpAs)
JOSpAs represent a group of HLA‑B27-associated pediatric rheumatic diseases characterized by inflammatory peripheral arthritis, enthesitis, sacroilitis, absence of RF and ANA, and tendency for acute ocular inflammation and variable mucocutaneous lesions.8 This group includes juvenile ankylosing spondylitis, reactive arthritis, inflammatory bowel disease (IBD)-related arthritis, and psoriatic arthritis.
Juvenile ankylosing spondylitis is a chronic arthropathy that predominantly affects boys after the age of 10 years.24,25 Patients have a history of back pain, radiographic involvement of the sacroiliac and sometimes the lumbosacral spine, and peripheral arthritis together with enthesitis. More than 91% of patients are HLA‑B27 positive.24,25 Recurrent attacks of symptomatic acute anterior uveitis can develop, in contrast to the chronic asymptomatic iridocyclitis of JIA.25 Uveitis is seen in 10%–15% of patients and the attacks typically involve only 1 eye at a time, although the inflammation can switch back and forth between eyes. The typical presentation is of severe acute anterior uveitis, often with fibrin and hypopyon (Figure 2).1,24
Juvenile reactive arthritis and IBD-related arthritis are uncommon in children.26–28 Reactive arthritis in children has the same pathogenic and clinical characteristics as in adults, with a classic triad of nonspecific urethritis, polyarthritis, and conjunctival inflammation accompanied by iritis.29 The uveitis attacks are typically unilateral, resembling those seen with ankylosing spondylitis. IBD-associated arthritis is typically mild, pauciarticular, and affecting large joints. Less frequently, spondylitis and sacroilitis are seen, often associated with the presence of HLA‑B27.28 Children with IBD can develop any type of ocular inflammation, including acute or chronic anterior uveitis, scleritis, CME, or retinal vasculitis.30 Chronic uveitis is more commonly seen in children with peripheral joint disease.26,-29
Juvenile psoriatic arthritis is defined as arthritis occurring with psoriasis, dactylitis, or nail pitting, or a family history of psoriasis.31 If psoriatic arthritis is seen before 3 years of age, it clinically resembles the antinuclear antibody (ANA)-positive oligoarthritis subtype of JIA. Anterior uveitis occurs in approximately 10%–20%. When the onset of arthritis is in early childhood, the uveitis is typically chronic.32 When the arthritis develops in later childhood, symptomatic, recurrent, acute, anterior uveitis is typical. There is no specific HLA association except in those children with sacroilitis, who are likely to be HLA‑B27 positive. In most of these cases, visual prognosis is good.32
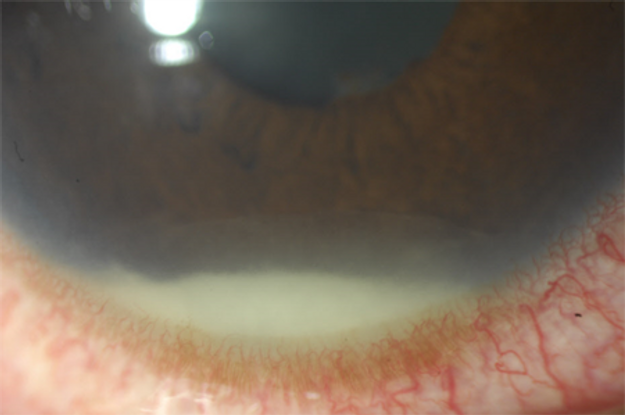
Figure 2. HLA‑B27 acute uveitis with intense circumciliary injection seen inferiorly and a hypopyon with overlying fibrin.
Tubulointerstitial Nephritis and Uveitis (TINU)
Acute tubulointerstitial nephritis is an under-recognized cause of acute kidney injury (AKI). It is estimated to account for 15%–20% of case of AKI and is a reported diagnosis in 2.8% of all renal biopsies.33 Drug-induced cases account for 70% of all cases and several different agents have been incriminated. It also can be idiopathic or secondary to autoimmune or infectious causes.33
Dobrin and colleagues34 first reported the association between uveitis and tubulointerstitial nephritis in 1975, when they described 2 adolescent girls with interstitial nephritis and bilateral anterior uveitis. Extensive investigation for an etiologic agent was unrewarding and neither patient could be placed in any existing diagnostic category.
TINU has been estimated to account for 1%–2% of all uveitis patients seen in tertiary referral centers.35,36 The initial descriptions noted a female-to-male ratio of 3:1 and a median age of 15 years without a racial predilection.37 However, more recent literature suggests a slight male predominance.38 TINU syndrome is strongly associated with HLA- DQA1*01, HLA-DQB1*05, and HLA-DRB1*01. The association with HLA- DRB1*0102 is one of the highest reported for any disease.39
TINU is a systemic disease and patients can present with a variety of nonspecific signs and symptoms related to the renal disease. The most common initial symptoms are fever, weight loss, fatigue, and malaise. Other common initial symptoms include anorexia, weakness, abdominal or flank pain, and arthralgias or myalgias.35,37 Screening for TINU includes assessment of urinary β2 microglobulin, which can remain abnormal for months after the other abnormalities in the urinalysis have resolved.35,38 Other work‑up includes analysis of renal function (BUN, creatinine), urinalysis for proteinuria, normoglycemic glucosuria, microhematuria, and sterile pyuria. Definitive diagnosis of the interstitial nephritis can require kidney biopsy. In most cases the cause is not identified, although it can develop after systemic use of drugs such as nonsteroidal anti-inflammatory agents and antibiotics.37
Ocular signs and symptoms can precede, develop concurrently with, or follow the onset of interstitial nephritis.40,41 Uveitis has been documented up to 2 months before and up to 14 months after the onset of systemic symptoms. In patients who developed ocular symptoms after the onset of systemic symptoms, the median time to onset of ocular symptoms is 3 months after the onset of systemic symptoms.37
The most common ocular complaints are pain, redness, decreased vision, and light sensitivity. The uveitis is predominantly anterior (80%), bilateral (77%), and nongranulomatous, although granulomatous inflammation can also occur. It typically presents as bilateral, simultaneous-onset, anterior uveitis, although posterior or panuveitis has been described in approximately 20% of cases.37 Vitritis, pars plana exudates, retinal vascular sheathing, intraretinal hemorrhages and exudates, focal chorioretinitis, and multifocal choroiditis have all been described.37,40
The pathogenesis of TINU syndrome remains unknown. Histology of renal biopsy specimens reveals cellular infiltration by T-cells and cell-mediated immunity has been implicated.42,43 Lymphokine abnormalities, presence of soluble IL‑2 receptors, and decreased CD4/CD8 ratio are other findings suggestive of the role of cell-mediated immunity.42,43
There are other conditions that can affect the kidneys and cause uveitis that must be considered in the differential diagnosis of TINU. These include sarcoidosis, post-streptococcal syndrome, Behçet disease, syphilis, and tuberculosis (TB). A thorough review of systems along with appropriate laboratory investigations helps in the diagnosis of TINU.
The prognosis of TINU is considered to be favorable, although the eye disease typically lingers longer than the renal disease. Mandeville and colleagues found that in the published literature until year 2000, 17% of 123 patients were treated with topical steroids alone, 80% were treated with systemic corticosteroids, and 9% were treated with immunomodulatory therapy.37 In a case series of 33 patients by Mackensen and colleagues, 36% were treated with topical corticosteroids alone.44 For the majority of the patients (64%), short-term systemic corticosteroid treatment was instituted. None of the patients received other systemic immunosuppressive agents.44 Steroid therapy is also administered for renal disease, when the impairment is severe or prolonged, although mild renal disease is thought to resolve spontaneously.45,46 In some instances, steroid-sparing immunomodulators such as mycophenolate mofetil and cyclosporine have been used.45,46
TINU is likely to be more common than once thought, and might be under-diagnosed. As testing for this entity becomes more common, the published prevalence is likely to be higher than in the previous literature. Posterior involvement is more common than previously reported, and systemic immunomodulatory therapy is often required.
Early-Onset Sarcoidosis/Blau Syndrome
Early-onset sarcoidosis (EOS) is seen in children less than 5 years old and is relatively rare.47 In 1985, Blau syndrome (BS) was described simultaneously by Blau and Jabs48,49 as a familial, granulomatous, inflammatory disease with an autosomal-dominant inheritance pattern. In 2001, Miceli-Richard and colleagues identified 3 missense mutations in the nucleotide-binding domain of CARD15/NOD2 in 4 French and German families with BS.50 Their findings indicated that, in addition to Crohn disease, CARD15 is involved in the susceptibility to BS. Similar studies in EOS resulted in the association of this disease with CARD15/NOD2 mutation as well.51,52 It became clear that BS and EOS are the familial and sporadic forms, respectively, of the same disease.
BS presents in early childhood as a triad of granulomatous dermatitis, polyarthritis, and uveitis.47 Typically, skin rash is the first symptom to appear, usually in the first year of life. Between the ages of 2–4 years, a boggy polyarthritis is observed. Finally, uveitis develops in 60%–80% of patients at about 4 years of age.47 Despite the increasing use of genetic testing, the real prevalence of BS and EOS remains unknown. Rough incidence data derived from analysis of a total of 48 Danish childhood-onset sarcoidosis patients during the period 1979–1994 estimated the incidence of 0.06 per 100,000 person-years for those younger than 5 years of age and 0.10 per 100,000 for those 5 to 9 years of age.50
The most frequent appearance of the skin rash is fine, scaly, erythematous, and maculopapular, appearing on the trunk and extremities in an infant. The rash is often misdiagnosed as atopic dermatitis, or when desquamating, as ichthyosis vulgaris.46 Histology reveals noncaseating granulomas with epithelioid and multinucleated giant cells. The symmetric polyarthritis seen in BS and EOS mainly involves the metacarpal-phalangeal, metatarsal-phalangeal and proximal-interphalangeal joints of hands and feet, wrists, knees, ankles, and rarely, elbows. Complications can occur with deformity, ankylosis, or bone erosions.47 Tenosynovitis is also a characteristic feature, and the arthritis in the small joints of the hands is associated with symmetric, hypertrophic tenosynovitis and tenosynovial cysts.
Noncaseating granulomatous synovitis with typical epithelioid and multinucleated giant cells is detectable histologically in biopsies of involved joints.47 Granulomatous uveitis is a frequent and severe manifestation of BS and EOS.54 It can be bilateral with chronic or recurrent involvement, affecting both anterior and/or posterior segments with iridocyclitis (Figure 3), vitritis, chorioretinitis, periphlebitis, macular edema, vein occlusion, and optic neuropathy. A clinical picture similar to idiopathic retinal vasculitis, aneurysms, and neuroretinitis (IRVAN) has been described in a patient with BS.54,55 Bilateral, lower motor-neuron facial palsy and bilateral hearing loss have also been reported in patients with EOS.56
BS and EOS are characterized by the presence of noncaseating epithelioid and giant-cell granulomas in affected tissues. The morphologic characteristics of these granulomas are similar to those found in sarcoidosis, which is not associated with NOD2 mutations. NOD2/CARD15 is mapped to chromosome 16q12 and encodes the multidomain cytosolic NOD2 protein, which is a key regulator of innate immunity because it functions as a bacterial detector with subsequent inflammatory signaling responses.57–59 Due to this role and its protective function against mycobacteria, an infectious-trigger hypothesis has been formulated to explain the pathogenesis of BS and EOS, although evidence is very limited.60 Specific laboratory investigations, a thorough review of systems, and histopathologic examination of tissue, along with genetic testing when applicable, help aid in correct diagnosis.
Due to the rarity and heterogeneity of BS and EOS, there are no specific therapeutic suggestions. The inflammation is treated as in other chronic uveitis, with consideration of initial systemic corticosteroid therapy, typically followed by systemic immunomodulatory therapy with antimetabolites and/or biologic agents such as adalimumab and infliximab.54,61,62

Figure 3. Large, greasy, "mutton-fat" keratic precipitates in a teenager with biopsy-proven sarcoidosis. Even the smaller KP and deposits between the large KP have a greasy appearance.
Infectious Anterior Uveitis
Infectious uveitis in children accounts for 6%–33% of all cases of pediatric uveitis.63–65 It can be caused by reactivation of a congenital infection or can be due to an acquired infection in childhood. Because treatment of infectious uveitis differs completely from that due to noninfectious etiologies and the treatment for noninfectious uveitis can make infections significantly worse, it is imperative to distinguish between the 2 early in the course of the disease.
Until recently there were very few systematic studies of infectious uveitis in children; 345 children with uveitis presenting to a tertiary referral center in the Netherlands from 1995 through 2010 were evaluated for infectious causes by serology and aqueous humor analysis.66 A diagnosis of infectious uveitis was based on a combination of clinical, laboratory, and imaging findings and was established in 17% of children. Fuchs heterochromic iridocyclitis (FHI) was classified as infectious uveitis because of the established relationship between FHI and rubella virus. Ocular toxoplasmosis was the most frequently diagnosed infectious disorder, representing 36 of 60 cases (60%), and all patients had chorioretinal lesions.66 The second-most prevalent cause of infectious uveitis in this cohort was viral, representing 18 of the 60 cases (30%), with VZV as the most common pathogen, affecting 7 of the 18 viral cases (39%). Five VZV patients had anterior uveitis and 2 had acute retinal necrosis (ARN). Three of the VZV-associated anterior uveitis patients presented with keratouveitis, 2 of whom had recently had chickenpox.66 Three children, all of whom were immunocompetent, had congenital CMV. One CMV patient presented with anterior uveitis and a cataract and the other 2 had chorioretinal scars.
The study authors concluded that confirming viral uveitis can facilitate timely and appropriate treatment and recommended aqueous humor analysis in every child with sight-threatening uveitis of unknown cause as well as unclear cases in which an infectious cause is suspected despite the requirement for general anesthesia in younger children.66
The herpes viridae are a large family of DNA viruses that can be found in nearly all animal species, 8 of which are predominantly found in human herpes simplex virus (HSV) 1 and 2, varicella zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV) and human herpes virus (HHV) 6, 7, and 8.67,68 HSV1 and HSV2 are transmitted by close personal contact. HSV1 causes labial infections and HSV2 is typically transmitted sexually. VZV causes 2 distinct diseases: Varicella, or chicken pox, is seen as primary infection and causes a self-limiting disease in childhood, whereas zoster occurs after reactivation of latent VZV virus in the sensory ganglia.67,68 Iritis and glaucoma secondary to herpes virus infections of the anterior uvea were initially characterized in the 1980s.69 Iritis and trabeculitis secondary to HSV or VZV can occur with or without noticeable corneal lesions. In patients with VZV reactivation, vesicular rash is seen in a dermatomal distribution in immunocompetent hosts.70 Cutaneous involvement at the tip of the nose indicates nasociliary nerve involvement (Hutchinson's sign) and might be associated with a greater likelihood of ocular involvement. However, absence of this sign in no way precludes ocular involvement, and all patients with zoster require a complete eye examination.
Patients can present with redness, pain, photophobia, and blurred vision. Mild to moderate anterior-chamber reaction might be seen along with white or stellate KP. The KP can be diffusely distributed over the corneal endothelium not respecting Arlt's triangle.70 They can also be larger and granulomatous appearing, often in the central cornea. Sectoral or patchy iris atrophy is characteristically seen and is best demonstrated with retroillumination at the slit-lamp (Figure 4).71
Histologic studies have demonstrated perineuritis and perivasculitis with herpetic ocular disease, and occlusive vasculitis has been implicated as a cause of iris atrophy.72 Trabeculitis with resultant ocular hypertension and intraocular pressures to the mid‑50s is also characteristic of herpetic iritis. A dilated fundus exam to exclude herpetic retinitis is required. Treatment of herpetic iritis involves institution of systemic antivirals along with low-dose topical-corticosteroid therapy.73,74
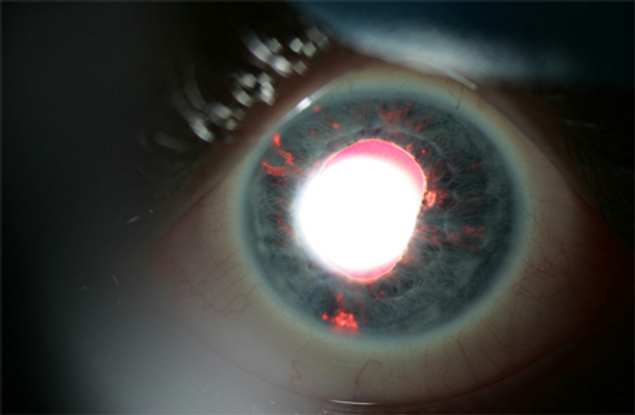
Figure 4. Patchy iris atrophy of herpetic iridocyclitis, best visualized with a broad, short beam under coaxial illumination at low power.
Fuchs Heterochromic Iridocyclitis
FHI is a chronic, nongranulomatous, typically unilateral iridocyclitis whose etiology remained unknown for several decades. Several hypotheses existed as to its etiology75,76 until recently, when improvements in the ability to detect infectious organisms using quantitative antibody and polymerase chain reaction (PCR) studies yielded new evidence of a strong association between the rubella virus and FHI.77,78 FHI is estimated to represent 2% to 7% of all cases of anterior uveitis in North America, with a similar prevalence seen worldwide.79 There is no sex predilection. The uveitis typically firsts presents in young adulthood, but there can be a long delay in accurate diagnosis.80,81
The condition is predominantly unilateral, but is seen bilaterally in 10% of cases. It is characterized by a quiet-looking eye on external eye exam, small stellate keratic precipitates scattered through the endothelium, variable AC cell and flare, anterior vitreous cells, iris atrophy with or without heterochromia, abnormal angle vessels on gonioscopy, and lack of posterior synechiae. The development of posterior subcapsular cataract is common.82,83 The iris of the affected eye is most often hypochromic, especially in patients with brown irides, but can also be hyperchromic in patients with blue irides owing to profound atrophy of the anterior iris stroma revealing the darkly pigmented iris neuroectoderm82,83 (Figure 5). The radial iris vessels are often quite prominent and can extend to overlie the trabecular meshwork. The Amsler sign can appear on anterior chamber paracentesis and is characterized by the appearance of filament-like blood in the anterior chamber. Peripheral anterior synechiae and posterior synechiae are very rare in the absence of surgery. The presenting complaint can arise from decreased vision from cataracts or due to visual disturbances from vitreous floaters.82,83 FHI can sometimes be initially detected in asymptomatic patients on routine eye examination.
Although this condition is usually associated with an excellent prognosis, glaucoma control can be difficult.84 Patients are often treated with corticosteroid eye drops, but they typically do not result in objective improvement in inflammation or help with symptom relief.75,83 In fact, these drops can hasten cataract formation and worsen glaucoma, and most uveitis specialists do not use corticosteroids to treat the uveitis of FHI.
Recent reports have shown an association between rubella virus and FHI. In 2006 deGroot Minjes et al. evaluated intraocular immunoglobulin G production using Goldmann-Witmer coefficient (GWC) against rubella virus (RV), HSV, VZV, and Toxoplasma gondii in the aqueous humor of 14 patients with FHI, 13 control subjects with herpetic anterior uveitis, and 19 control subjects with ocular toxoplasmosis.77 Intraocular antibody production (GWC > 3) against RV was found in 13 of 14 patients (93%) with FHI. Intraocular antibody production against HSV, VZV, or T gondii was not detected in these patients and none of the control subjects with herpetic anterior uveitis or toxoplasma chorioretinitis had a positive GWC for rubella virus. Those authors concluded that rubella virus, but not HSV, VZV, or T gondii, is associated with FHI.77
Subsequently Birnbaum et al.78 found a reduction in FHI cases in the United States after institution of the rubella vaccination program in the US. Those authors found that FHI was less common in patients born in the US since the introduction of the US rubella vaccination program, with a corresponding increase in percentage of foreign-born cases, providing epidemiologic support for the rubella virus as a cause of FHI.78
FHI is a clinical diagnosis and there are no laboratory tests to confirm the diagnosis. Differential diagnosis includes conditions that present with iris heterochromia such as Horner syndrome, Duane syndrome, or Waardenberg syndrome, but these conditions do not present with anterior chamber inflammation. Other conditions such as herpetic iritis and Posner-Schlossman syndrome have anterior uveitis with elevated IOP. However, Posner-Schlossman syndrome presents with acute episodes of symptomatic elevations of high IOP and mild inflammation rather than the chronic inflammation and gradual increase in IOP of patients with FHI. Patients with herpetic iritis tend to have high IOP at presentation rather than the later development of elevated IOP as seen in FHI. In addition, the iris atrophy of herpetic disease is much different than that of FHI, as described above.
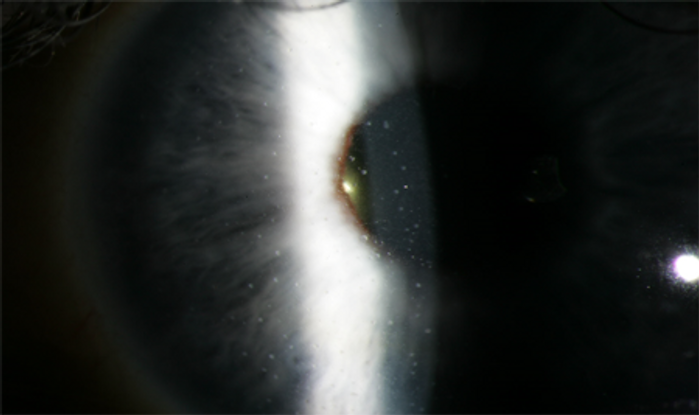
Figure 5. Small, white, stellate KP diffusely distributed in a patient with Fuch heterochromic iridocyclitis. The iris stroma is also atrophic.
Postinfectious Autoimmune Uveitis
Uveitis occurring 2 weeks after a streptococcal pharyngitis was first reported by Cokington and Han in 1991.85 Since then there have been several reports of uveitis presenting as an immune-mediated sequelae to group A streptococcal infection.86–89 The syndrome is more common in younger patients and usually presents 1 to 6 weeks after onset of systemic symptoms.88–90 The disease presents with bilateral uveitis and is associated with elevated serum antistreptolysin O (ASO) titers in more than 95% of cases.85,88,89 Titers are normally elevated 1 week after infection with group A Streptococcus and peak between 3 to 6 weeks. A decrease begins after 6 to 8 weeks, but some patients maintain elevated levels for longer periods of time.85,88,89
Rehman and colleagues identified 10 consecutive cases of post-streptococcal syndrome uveitis.89 All had bilateral nongranulomatous inflammation and elevated ASO titers. Birnbaum et al90 evaluated a series of patients with simultaneous-onset nongranulomatous, bilateral, acute anterior uveitis (BAAU) at a tertiary uveitis referral center between 1990 and 2010. Those authors found that patients who presented with BAAU composed approximately 1% of the total number of new cases of uveitis seen at the center and 6% of patients with nongranulomatous acute anterior uveitis. Children younger than 17 years of age composed 25% of the patients with BAAU. Patients with BAAU were found to be younger than the cohort of other new patients with uveitis evaluated during the same time period and the cohort of new patients with unilateral acute anterior uveitis. The most common association was postinfectious or drug-induced uveitis (52%). The median duration from the onset of illness to the onset of eye symptoms for patients with postinfectious uveitis was 7 days (range 1–30 days).90
Management involves treatment of the uveitis as well as monitoring of ASO titers. A rising antistreptococcal lysin‑O titer can indicate repeat clinical or subclinical streptococcal infection and require further antibiotic therapy.91
Masquerade Anterior Uveitis
Masquerade syndromes comprise a group of disorders that either simulate a chronic idiopathic uveitis or have an underlying nonimmune disease that resembles intraocular inflammation. In children, the most common masquerade syndromes that present as anterior uveitis are leukemia, retinoblastoma, and juvenile xanthogranuloma.
Leukemia
The leukemias are the most common malignant neoplasms in childhood, accounting for approximately 31% of all malignancies in children less than 15 years of age, with an annual incidence of 4.5 cases per 100,000 children in the US.92 They are characterized by diffuse replacement of the bone marrow by neoplastic cells. The most widely used and clinically useful classification divides leukemia into acute and chronic forms.92 The acute leukemias are characterized by replacement of the bone marrow with very immature cells called blasts. Chronic leukemias are associated, at least initially, with well-differentiated (mature) leukocytes.
Two major variants of acute and chronic leukemia are recognized: lymphocytic and myelogenous. Acute lymphoblastic leukemia (ALL) accounts for approximately 77% of cases of childhood leukemia, acute myelogenous leukemia (AML) for 11%, chronic myelogenous leukemia (CML) for 2%–3%, and juvenile myelomonocytic leukemia (JMML) for 1%–2%.92 Anterior segment involvement most commonly occurs in patients with ALL and less commonly with CLL and AML.93,94 Patients can present with unilateral or bilateral symptoms with or without conjunctival injection. Often there is appearance of iridocyclitis with a pseudohypopyon or a spontaneous hyphema.95 The pseudohypopyon is composed of shaggy, free-floating material that fails to completely settle, giving rise to a characteristic "lumpy bumpy" appearance. This tumor "hypopyon" is creamy white in color and can initially partially respond to topical corticosteroids. Diffuse iris infiltration by leukemic cells is characterized by iris discoloration or masses, and there can be heterochromia iridis.95,96 Nodular involvement is seen as ill-defined iris masses extending into the pupillary margin. Infiltration of the trabecular meshwork or choroidal infiltration can result in glaucoma.97
Diagnosis is made by anterior chamber paracentesis and cytologic examination of the aqueous humor for malignant cells.95–97 Prompt referral to an oncologist can be lifesaving.
Retinoblastoma
Retinoblastoma is the most common primary cancer to affect the eyes of children, with 250–300 cases per year in the US and 6,000–8,000 per year worldwide.98 Children less than 3 years of age are predominantly affected and the tumor rarely presents over the age of 5 years. It affects males and females equally. Approximately 60% of cases are unilateral. The retinoblastoma susceptibility gene, RB1, is a tumor-suppressing gene, and children diagnosed with retinoblastoma can have a mutation that is germline or somatic. Germline mutations account for one-third of cases, and these patients tend to have bilateral and multifocal disease. The most common modes of presentation are leukocoria and strabismus.98 Patients can present with signs of intraocular inflammation, either true inflammation secondary to tumor necrosis or simulated inflammation with tumor cells in the anterior chamber being mistaken for inflammatory cells.
A case series by Stafford and colleagues reported that nearly 40% of patients with retinoblastoma were initially misdiagnosed as having uveitis.99 Croxatto et al. reported a case of a 9‑year-old girl who presented with a pseudohypopyon of 6 months' duration in which chalky-white flocculent material was found in the anterior chamber.100 Whitish nodules were seen on the iris and an iris biopsy was performed, which was read as granulomatous iritis. The patient's condition worsened and eventual aqueous tap cytology revealed retinoblastoma cells. The eye was enucleated and histopathology revealed diffuse infiltrating retinoblastoma.100
Because retinoblastoma is a common malignancy in childhood, any patient presenting with strabismus, leukocoria, or anterior uveitis must have a detailed ophthalmic exam, including a dilated fundus exam and an examination under anesthesia when indicated. A thorough review of systems including family history is imperative. Enucleation remains the definitive treatment of intraocular retinoblastoma, particularly in unilateral disease.101 Other treatment modalities include systemic chemotherapy and intra-arterial chemotherapy. For small tumors, cryopexy, laser photocoagulation, hyperthermia, and plaque irradiation can be considered.102
Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) is predominantly a skin disorder of the young characterized by multiple cutaneous papules.103 Extracutaneous lesions can involve the eye and adnexa, lungs, spleen, gastrointestinal tract, and pericardium. Ocular structures that can be involved are the uveal tract including iris, ciliary body, and choroid; eyelids; the epibulbar area; cornea; conjunctiva; optic nerve; retina; and the orbit.104,105 Most patients with JXG are less than 2 years of age at presentation and it has been reported that most patients with ocular involvement present within the first year of life. Ocular lesions are typically unilateral. Iris infiltration represents the most frequent ophthalmic manifestation and is characterized by fleshy iris nodules or diffuse infiltration.103 The lesion is highly vascular and can cause spontaneous hyphemia. Patients can present with heterochromia iridis. Occasionally, patients present with redness and pain along with anterior chamber flare and cells mimicking iridocyclitis.106
Secondary glaucoma can occur due to infiltration of the angle structures or blockage of the trabecular meshwork by blood in the anterior chamber.
Histologically, JXG is characterized by infiltration by foamy histiocytes, Touton and other multinucleated giant cells, and various other inflammatory cells. Staining with Oil Red O demonstrates neutral fat within the histiocytes and giant cells. There is less tendency for the infiltrating iris cells to have the foamy, lipid-laden cytoplasm characteristically seen in cutaneous lesions.108
JXG is a rare disorder, and many ophthalmologists are insufficiently familiar with this condition, leading to a delay in diagnosis. Therefore, any young infant presenting with unilateral heterochromia iridis, spontaneous hyphemia, secondary glaucoma, and ocular inflammation must be suspected of having JXG. Aqueous paracentesis with or without an iris biopsy can aid in making this diagnosis.107
In children with only cutaneous lesions, no treatment is indicated, although close monitoring for possible development of ocular lesions is recommended. Early treatment of JXG uveal lesions without glaucoma is with topical or subconjunctival steroids.108 If initial therapy fails, systemic steroids, low-dose irradiation, or a combination of systemic steroids and irradiation should be considered.109
Traumatic Uveitis
Uveitis can develop after minor, blunt, nonpenetrating ocular trauma. If the injury is minor, but severe inflammation is seen, an underlying predisposition such as HLA‑B27 positivity might exist. Patients present with miosis, ciliary flush, and decreased intraocular pressure. Hyphemia might also be present. Rosenbaum et al. analyzed the records of 496 patients seen in the uveitis clinic of a tertiary referral center to evaluate the role of nonpenetrating trauma in initiating uveitis;110 24 of the 496 patients (4.8%) suspected that their intraocular inflammation was related to previous nonpenetrating trauma. Patients with post-traumatic uveitis were usually male (79%), younger (31 +/- 16 years) than the average patient examined in the uveitis clinic, and more likely to have unilateral disease. In 42% of patients, the trauma was work-related. Bilateral inflammation was seen in one‑third of patients and 71% had a considerable degree of inflammation posterior to the lens. Many patients also had an identifiable cause of uveitis such as ankylosing spondylitis, reactive arthritis, or sarcoidosis, but most patients had no known predisposition.110
Traumatic anterior uveitis is treated with topical corticosteroids and cycloplegics. In severe inflammation or if penetrating injury is suspect, intraocular foreign body should be ruled out.
Penetrating ocular injuries can result in severe uveitis. In some cases, the penetrating injury is so small that its entrance can be missed. Persistent unilateral iridocyclitis after penetrating injury of the cornea should arouse suspicion of occult foreign material in the anterior chamber angle or in the ciliary body, warranting gonioscopic exam and imaging, which can include x‑rays, CT, and anterior segment ultrasonography.
Idiopathic Uveitis
When no systemic cause for uveitis is found, a diagnosis of idiopathic uveitis is made. In the multicenter series of pediatric uveitis by Smith et al.4 that evaluated 527 pediatric uveitis patients, the leading diagnoses were idiopathic uveitis (28.8%), juvenile idiopathic arthritis-associated uveitis (20.9%), and pars planitis (17.1%). BenEzra et al63 found that 25.4% of 821 children and adolescents with uveitis had idiopathic disease. Idiopathic uveitis should be considered a diagnosis of exclusion after all systemic causes for ocular inflammation have been ruled out.
Classification, Diagnosis, Imaging
A comprehensive history and review of systems is of paramount importance in the evaluation of a patient with uveitis. Every patient needs to be asked about the current episode of uveitis, past episodes, systemic diseases, medications, travel and other exposures, family history, prior surgeries or hospitalizations, and a detailed review of systems. Remember that patients will not typically volunteer systemic symptoms to an ophthalmologist and questioning must be detailed and specific. A differential diagnosis for uveitis is developed based on classification of uveitis, which requires a detailed history and examination.
Uveitis can be classified anatomically, based on timing, on whether it is clinically granulomatous or nongranulomatous, and on whether there are associated systemic features. Acute uveitis is sudden in onset and of limited duration, resolving within 3 months, whereas chronic uveitis is persistent, with relapse less than 3 months after discontinuation of treatment. Recurrent uveitis is characterized by repeated episodes of inflammation separated by periods of inactivity of at least 3 months without any treatment. Categorization can be further established based on whether the inflammation is severe or low-grade and whether inflammation is found unilaterally or bilaterally. Uveitis is also characterized based on whether it is clinically granulomatous or nongranulomatous and based on primary site of inflammation (anterior, intermediate, posterior, or panuveitis, in which the anterior chamber, vitreous and retina, or choroid are equally involved). Age, gender, racial background, travel, and occupational history are additional important clues to aid in diagnosis.
An accurate slit-lamp biomicroscopic exam, along with a detailed fundus exam, is helpful in narrowing the differential diagnosis. Every patient with anterior chamber inflammation must receive a dilated fundus exam to exclude inflammation within the posterior segment. Additionally, macular edema can occur with severe anterior chamber inflammation and will be missed if the fovea is not properly examined. Optical coherence tomography (OCT) of the macula can aid in detecting subtle changes or intraretinal cysts within the retina.
Most patients with uveitis are worked up for sarcoidosis, syphilis, and tuberculosis. Other testing is based on history and exam; for example, a child with chronic bilateral anterior uveitis would have ANA testing because JIA is a likely diagnosis, and a teenager with severe acute anterior uveitis in 1 eye would be tested for HLA‑B27. Lyme testing is useful in a Lyme endemic area or following history of tick bite and characteristic rash.111 There is limited utility in a "shot gun" approach to uveitis testing, and the positive predictive value of every test must be considered. Testing for sarcoidosis includes a chest x‑ray and might include obtaining angiotensin-converting enzyme (ACE) serum and lysozyme levels.112 Although CT chest is often obtained looking for sarcoidosis in older patients,113 it should probably not be used as a screening tool in pediatric patients, because of the radiation dose.114 Testing for TB can be performed via skin testing (PPD) or serum interferon gamma assay such as the QuantiFERON Gold. Syphilis testing includes both nontreponemal testing such as RPR and VDRL and treponemal testing such as fluorescent treponemal antibody absorption test (FTA-ABS) or Treponema pallidum particle agglutination (TPPA).
Complications
A significant proportion of children with moderate to severe uveitis can suffer from visual loss due to ocular complications. A series from the Netherlands115 in 2003 evaluated 123 children with anterior, intermediate, posterior, and panuveitis for specific causes of visual loss and characteristics associated with poor visual outcome. The anatomical location of uveitis was anterior in 36%, intermediate in 24%, posterior in 19%, and panuveitis in 21%. It was chronic in 83% and bilateral in 73%. Complications of uveitis developed in 76%. The most frequent complications were cataract in 35% and glaucoma in 19%.115 Of the patients with glaucoma, 48% had JIA. Optic disc edema was noted in 29% of patients and CME was detected in 17%. Intraocular surgery was required in 28%. The most frequent causes of blindness were macular scars and secondary glaucoma. The most frequent cause of visual impairment was CME.
The following year, a series of 148 children with uveitis in the US reported the cumulative proportion and visual significance of ocular complications.116 Of the 148 patients, 71% had bilateral uveitis. The most common anatomic diagnosis was anterior uveitis (30.4%). Idiopathic uveitis was the most common etiologic diagnosis (26.4%), followed by anterior uveitis associated with juvenile rheumatoid arthritis (JRA) (23.0%); 34% had 1 or more complications at time of uveitis diagnosis. The frequency of complications increased to 61.6% by 3 months, 69.4% at 6 months, 75.2% at 1 year after diagnosis, and 86.3% by 3 years. Each of the common complications of band keratopathy, cataract, posterior synechiae, glaucoma/hypertension, CME, and hypotony increased in frequency during the course of the study. The percentages of cases with these complications that were bilateral at diagnosis were 51.1% (band keratopathy), 50.8% (posterior synechiae), 43.5% (CME), 40.9% (cataract), 25.0% (glaucoma), and 5.0% (hypotony).116
Risks of the most common complications varied among types of uveitis. Anterior and intermediate uveitis had a higher risk of band keratopathy (p = 0.005), posterior and intermediate uveitis had a lower risk of posterior synechiae (p < 0.001), and intermediate uveitis had a higher risk of CME (p = 0.002). Patients with posterior uveitis were least likely to develop cataract (p = 0.007) and the risk of any complication was lowest for anterior uveitis.116
Cataract, typically posterior subcapsular, is a common complication, occurring in 21%–84% of JIA uveitis patients.20,117,118 Cataracts develop not only because of anterior chamber inflammation, but also due to corticosteroid use. In a series by Thorne et al., 97 children with JIA were evaluated over a median follow-up period of 4 years. Those authors found that use of topical corticosteroids was associated with cataract formation independent of uveitis activity.119 Using longitudinal data analysis and controlling for duration of uveitis, presence and degree of active uveitis, and concomitant use of other forms of corticosteroids in a time-updated fashion, treatment with ≤ 3 drops daily of topical corticosteroid was associated with an 87% lower risk of cataract formation compared with > 3 drops daily (relative risk, 0.13; 95% CI, 0.02–0.69; P = 0.02).
Local steroid therapy should be limited in children to prevent this significant complication and consideration given to early institution of systemic immunomodulatory therapy in children with chronic uveitis. The emphasis on strict perioperative control of inflammation along with newer surgical techniques has improved the outcomes of cataract surgery in patients with uveitis, but this complication should still be avoided if possible. Perioperative control of inflammation for at least 3 months prior to surgery and continuation of strict control of inflammation postoperatively is imperative for good surgical outcomes. Intraocular lens (IOL) implantation in children with uveitis has been a topic of much debate due to increased incidence of posterior capsular opacification and development of membranes with cocooning of the IOL, which can result in irreversible hypotony.
The gold standard in the past was to leave children with JIA uveitis undergoing cataract surgery aphakic, with postoperative contact lenses or aphakic glasses. More recently good results have been reported with posterior chamber IOL implantation for JIA patients who develop cataracts.120 Posterior capsulorhexis and anterior vitrectomy can additionally be performed after cataract extraction. If an IOL is to be placed, patient selection is critical. In any child with difficult-to-control inflammation, hypotony, significant fibrin and posterior synechiae, or compliance issues, strong consideration should be given to leaving the child aphakic, with later secondary IOL placement when conditions have been optimized.
Secondary glaucoma is seen in 19%–25% of children with uveitis.4,115,116 Initially, during an acute inflammatory episode, the intraocular pressure (IOP) can be low due to inflammation of the ciliary body and decreased production of aqueous. Elevation of IOP often develops later, as aqueous secretion improves or outflow facilities have sustained damage due to inflammation.121 Therapy includes topical drops, oral carbonic anhydrase inhibitors, and incisional surgery. Surgical techniques include filtering surgery with or without antimetabolites and valve drainage procedures.122 Laser trabeculoplasty is rarely recommended for uveitic glaucoma.
Band keratopathy is the deposition of calcium hydroxyapatite in the Bowman layer of the cornea. The deposition initially begins in the nasal and temporal cornea, within the palpebral fissure, and then spreads centrally if the intraocular inflammation is poorly controlled. The keratopathy has a Swiss-cheese appearance due to lack of deposition of calcium at the point of entry of the corneal nerves (Figure 1). It occurs more often in pediatric uveitis patients than in adults and can be seen in up to 70% of patients with JIA uveitis.20 The chelating agent disodium ethylenediamine tetra-acetic acid (EDTA) or excimer laser can be used to remove the deposition, although it tends to recur over time.
CME can occur as a complication of inflammation in any part of the eye (anterior, intermediate, posterior, or panuveitis). It can be treated with local corticosteroid injection (intra or periocular), systemic corticosteroids, and steroid-sparing agents. Long-term systemic corticosteroid therapy should be avoided in all patients because of risk of systemic side effects, and in children this is compounded by the risk of growth retardation.123,124 As stated above, local steroid therapy should be minimized in children because of the heightened risk of glaucoma and cataract in children as compared with adults and the potential for more significant issues with ocular surgery in young patients as compared with adults. Topical difluprednate ophthalmic emulsion 0.05%, although very effective in controlling inflammation, including CME, can cause marked elevations in IOP, especially in children.125,126
In a series by Sallam et al., 15 children with uveitic CME were treated with intravitreal triamcinolone acetonide (IVTA).127 Those authors noted resolution of CME in all the treated eyes with median time to resolution of 3 weeks (range 1–24 weeks). However, following initial response, CME relapsed in 31% after a median time of 7 months (range 3–13 months). The treatment was complicated by increased intraocular pressure, with an increase of more than 15 mm Hg in 31%, and 50% of eyes requiring ocular hypotensive therapy, with a median treatment duration of 33 weeks (range 1–69 weeks). Steroid-induced cataract was observed in 55% of phakic eyes.127
Management
Medical therapy for uveitis, including systemic immunomodulatory therapy, is covered thoroughly in another section, so only a brief overview is described below.
Corticosteroids form the mainstay of treatment early in the course of noninfectious uveitis. Topical corticosteroids are effective for early control of uveitis, but in patients who require prolonged therapy with more than minimal topical steroid therapy, a long-term corticosteroid-sparing immunomodulatory therapy plan is typically required.128,129 Local therapy with peribulbar and intravitreal corticosteroids has been used to treat uveitis, more commonly intermediate and posterior uveitis.128,129 Injectable or surgically implantable steroid-eluding devices such as the biodegradable dexamethasone implant130 and fluocinolone acetonide implant131 have been studied in pediatric anterior uveitis. Long-term systemic corticosteroids are associated with growth retardation, weight gain, hyperglycemia, osteoporosis, adrenal suppression, among other protean side-effects. Systemic corticosteroids can be used as a short-term bridge to immunosuppressive therapy in patients not controlled with topical therapy, but are not a safe-long term option.132
Immunosuppressive agents can be broadly divided into nonbiologic and biologic agents. Among the nonbiologics are antimetabolites (methotrexate, mycophenolate mofetil, and azathioprine), signal transduction inhibitors (cyclosporine), and alkylating agents (cyclophosphamide and chlorambucil). Biologic agents used in the treatment of pediatric uveitis include tumor necrosis factor (TNF) alpha inhibitors (adalimumab, infliximab, certolizumab, golimumab), interleukin (IL)‑1 inhibitor (anakinra), IL‑2 inhibitor (daclizumab), IL‑6 inhibitor (tocilizumab), T‑cell activation inhibitor (abatacept), and cluster differentiation (CD)‑20 inhibitor (rituximab).131 The American college of rheumatology, along with pediatric uveitis specialists in 2019, developed guidelines for monitoring and treatment of patients with JIA-associated uveitis. Methotrexate and the monoclonal antibody tumor necrosis factor inhibitors adalimumab and infliximab were recommended to prevent the ocular co-morbidities following chronic inflammation.23 Systemic immunomodulatory therapy in children usually requires close cooperation between the ophthalmologist and a pediatric rheumatologist or other physician with experience monitoring this type of therapy in children so as to maximize control of inflammation while minimizing both ocular and systemic side effects.
References
- Zierhut M, Michels H, Stubiger N, et al. Uveitis in children. Int Ophthalmol Clin. 2005; 45:135-156.
- Thorne JE, Suhler E, Skup M, et al. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol. 2016 Nov 1;134(11):1237-1245.
- Cunningham ET Uveitis in children. Ocul Immunol Inflamm. 2000;8:251-261.
- Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009 Aug;116(8):1544-51.
- Wolf MD, Lichter PR, Ragsdale CG. Prognostic factors in the uveitis of juvenile rheumatoid Ophthalmology. 1987;94:1242-1248.
- Kotaniemi K, Kaipiainen-Seppa¨nen O, Savolainen A, et al. A population-based study on uveitis in juvenile rheumatoid arthritis. Clin Exp Rheumatol. 1999;17:119-122.
- Petty RE, Smith JR, Rosenbaum Arthritis and uveitis in children: a pediatric rheumatology perspective. Am J Ophthalmol. 2003;135:879-884.
- Wu EY, Van Mater HA, Rabinovich CE. Juvenile Idiopathic Arthritis. In: Kliegman R, Stanton B, Schor N, St.Geme J, Behrman R, eds. Nelson Textbook of Pediatrics. 19th ed. Elsevier Health Sciences, 2011: 829-838.
- Gregory AC 2nd, Kempen JH, Daniel E, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Ophthalmology. 2013; 120:186-192.
- Wood Nomenclature and classification of arthritis in children. In: Munthe E, editor. The Care of Rheumatic Children. Basle: EULAR Publishers; 1978:47-50.
- Brewer EJ, Bass J, Cassidy JT, et al. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1973;23:712-719.
- Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
- Kanski JJ. Juvenile arthritis and uveitis. Surv Ophthalmol. 1990;34:253-267.
- Rosenberg AM. Uveitis associated with childhood rheumatic diseases. Curr Opin Rheumatol. 2002 Sep;14(5):542-7.
- Vitale AT, Graham E, de Boer JH. Juvenile idiopathic arthritis- associated uveitis: clinical features and complications, risk factors for severe course, and visual outcome. Ocul Immunol Inflamm. 2013 Dec;21(6):478-85.
- Keenan JD, Tessler HH, Goldstein DA. Granulomatous inflammation in juvenile idiopathic arthritis-associated uveitis. J AAPOS. 2008 Dec;12(6):546-50.
- Vastert SJ, Bhat P, Goldstein DA. Pathophysiology of JIA- associated Ocul Immunol Inflamm. 2014 Oct;22(5):414-23.
- Kalinina Ayuso V, Makhotkina N, van Tent-Hoeve M, et al. Pathogenesis of juvenile idiopathic arthritis associated uveitis: the known and unknown. Surv Ophthalmol. 2014 Sep-Oct;59(5):517-31.
- Rosenberg AM, Oen KG. The relationship between ocular and articular disease activity in children with juvenile rheumatoid arthritis and associated uveitis. Arthritis Rheum. 1986;29:797-800.
- Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS. Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated uveitis. Ophthalmology. 1997 Feb;104(2):236-44.
- Levy-Clarke GA, Nussenblatt RB, Smith JA. Management of chronic pediatric uveitis. Curr Opin Ophthalmol. 2005; 16:281-288.
- American Academy of Pediatrics Section on Rheumatology and Section on Ophthalmology: Guidelines for ophthalmologic examinations in children with juvenile rheumatoid arthritis. Pediatrics. 1993 Aug;92(2):295-296.
- Angeles-Han ST, Ringold S, Beukelman T, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Screening, Monitoring, and Treatment of Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Rheumatol. 2019 Jun;71(6):864-877.
- Calabro JJ. Clinical aspects of juvenile and adult ankylosing spondylitis. Br J Rheumatol. 1983 Nov;22(4 Suppl 2):104-109.
- Schaller JG. Ankylosing spondylitis of childhood onset. Arthritis Rheum. 1977 Mar;20(2 Suppl):398-401.
- Rosenberg AM, Petty RE. Reiter's disease in children. Am J Dis Child. 1979 Apr;133(4):394-8.
- Iveson JM, Nanda BS, Hancock JA, Pownall PJ, Wright Reiter's disease in three boys. Ann Rheum Dis. 1975 Aug;34(4):364-368.
- Lindsley CB, Schaller JG. Arthritis associated with inflammatory bowel disease in children. J Pediatr. 1974 Jan;84(1):16-20.
- Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014 Apr-May;13(4-5):546-9.
- Ernst BB, Lowder CY, Meisler DM, Gutman Posterior segment manifestations of inflammatory bowel disease. Ophthalmology. 1991; 98: 1272-80.
- Southwood TR, Petty RE, Malleson PN, Delgado EA, Hunt DW, Wood B, Schroeder ML. Psoriatic arthritis in children. Arthritis Rheum. 1989 Aug;32(8):1007-13.
- Hafner R, Michels H. Psoriatic arthritis in children. Curr Opin Rheumatol. 1996;8:467-
- Raghavan R, Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol. 2014 Sep;82(3):149-162.
- Dobrin RS, Vernier RL, Fish FJ. Acute eosinophilic interstitial nephritis and renal failure with bone marrow-lymph node granulomas and anterior Am J Med. 1975; 596:325-333.
- Rosenbaum Bilateral anterior uveitis and interstitial nephritis. Am J Ophthalmol. 1988; 105:534-537.
- Kump LI, Cervantes-Castaneda RA, Androudi SN, Foster CS. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005; 112:1287-1292.
- Mandeville JT, Levinson RD, Holland GN. The tubulointerstitial nephritis and uveitis Surv Ophthalmol. 2001; 46:195-208.
- Howarth L, Gilbert RD, Bass P, Deshpande Tubulointerstitial nephritis and uveitis in monozygotic twin boys. Pediatr Nephrol. 2004; 19:917-919.
- Levinson RD, Park MS, Rikkers SM, et al. Strong associations between specific HLA-DQ and HLA-DR alleles and the tubulointerstitial nephritis and uveitis syndrome. Invest Ophthalmol Vis Sci. 2003; 44:653-
- Mackensen F, Billing H. Tubulointerstitial nephritis and uveitis syndrome. Curr Opin Ophthalmol. 2009 Nov;20(6):525-31.
- Gafter U, Ben Basat M, Zevin D, et al: Anterior uveitis, a presenting symptom in acute interstitial Nephron. 42: 249-51, 1986.
- Gafter U, Kalechman Y, Zevin D, et al: Tubulointerstitial nephritis and uveitis: association with suppressed cellular immunity. Nephrol Dial Transplant. 8:821-6,
- Yoshioka K, Takemura T, Kanasaki M, et al: Acute interstitial nephritis and uveitis syndrome: activated immune cell infiltration in the Pediatr Nephrol. 5:232-4, 1991.
- Mackensen F, Smith JR, Rosenbaum Enhanced recognition, treatment, and prognosis of tubulointerstitial nephritis and uveitis syndrome. Ophthalmology. 2007; 114:995-999.
- Vohra S, Eddy A, Levin AV, et al. Tubulointerstitial nephritis and uveitis in children and adolescents. Four new cases and a review of the literature. Pediatr Nephrol. 1999; 13:426-432.
- Kobayashi Y, Honda M, Yoshikawa N, Ito H. Acute tubulointerstitial nephritis in Japanese children. Clin Nephrol. 2000; 54:191-197.
- Wouters CH, Maes A, Foley KP, Bertin J, Rose CD. Blau syndrome, the prototypic auto-inflammatory granulomatous disease. Pediatr Rheumatol Online J. 2014 Aug 6;12:33.
- Blau EB: Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985, 107:689-693.
- Jabs DA, Houk JL, Bias WB, Arnett FC: Familial granulomatous synovitis, uveitis, and cranial neuropathies. Am J Med. 1985, 78:801-804.
- Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau Nat Genet. 2001, 29:19-20.
- Kanazawa N, Matsushima S, Kambe N, Tachibana T, Nagai S, Miyachi Presence of a sporadic case of systemic granulomatosis syndrome with a CARD15 mutation. J Invest Dermatol. 2004, 122:851-852.
- Rose CD, Doyle TM, McIlvain-Simpson G, et al. Blau syndrome mutation of CARD15/NOD2 in sporadic early onset granulomatous arthritis. J Rheumatol. 2005 Feb;32(2):373-375.
- Hoffmann AL, Milman N, Byg KE. Childhood sarcoidosis in Denmark 1979-1994: incidence, clinical features and laboratory results at presentation in 48 Acta Paediatr. 2004;93:30-36.
- Raiji VR, MillerMM, Jung LK. Uveitis in Blau syndrome from a de novo mutation of the NOD2/CARD15 gene. J AAPOS. 2011;15:205-207.
- Amin SR, Pulido JS. Retinal vasculitis, aneurysms, and neovascularization in Blau syndrome. JAMA Ophthalmol. 2013;131:677-680.
- Vaphiades MS, Eggenberger E. Childhood sarcoidosis. J Neuroophthalmol. 1998 Jun;18(2):99-101. PubMed PMID:
- Chen G, Shaw MH, Kim YG, Nuñez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365-398.
- Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9-23.
- Moreira LO, Zamboni DS. NOD1 and NOD2 signaling in infection and inflammation. Front Immunol. 2012;3:328.
- Caso F, Wouters CH, Rose CD, et al. Blau syndrome and latent tubercular infection: an unresolved partnership. Int J Rheum Dis. 2014;17: 586-7.
- Chauhan K, Michet C. A case of Blau syndrome. Case Rep Rheumatol. 2014;2014: 216056.
- Milman N, Andersen CB, Hansen A, et al. Favourable effect of TNF-alpha inhibitor (infliximab) on Blau syndrome in monozygotic twins with a de novo CARD15 mutation. APMIS. 2006;114:912-919.
- BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. 2005;89:444-448.
- Kump LI, Cervantes-Castaneda RA, Androudi SN, et al. Analysis of pediatric uveitis cases at a tertiary referral center. Ophthalmology. 2005;112:1287-92.
- Smith JA, Mackensen F, Sen HN, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. 2009;116:1544-1551.
- Hettinga YM, de Groot-Mijnes JD, Rothova A, de Boer JH. Infectious involvement in a tertiary center pediatric uveitis cohort. Br J Ophthalmol. 2015 Jan;99(1):103-107.
- Kim SR, Khan F, Ramirez-Fort MK, Downing C, Tyring Varicella zoster: an update on current treatment options and future perspectives. Expert Opin Pharmacother. 2014 Jan;15(1):61-71.
- Hennig T, O'Hare Viruses and the nuclear envelope. Curr Opin Cell Biol. 2015 Jun 26;34:113-121.
- Robin JB, Steigner JB, Kaufman HE. Progressive herpetic corneal endotheliitis. Am J Ophthalmol. 1985 Aug 15;100(2):336-337.
- Siverio Júnior CD, Imai Y, Cunningham ET Diagnosis and management of herpetic anterior uveitis. Int Ophthalmol Clin. 2002 Winter;42(1):43-48.
- Van der Lelij A, Ooijman FM, Kijlstra A, Rothova A. Anterior uveitis with sectoral iris atrophy in the absence of keratitis: a distinct clinical entity among herpetic eye diseases. Ophthalmology. 2000 Jun;107(6):1164-1170.
- Naumann G, Gass JD, Font RL. Histopathology of herpes zoster ophthalmicus. Am J Ophthalmol. 1968 Apr;65(4):533-541.
- Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994 Dec;101(12):1883-1895.
- Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994 Dec;101(12):1871-1882.
- Mohamed Q, Zamir E. Update on Fuchs’ uveitis syndrome. Curr Opin Ophthalmol. 2005;16:356-363.
- La Hey E, de Jong PT, Kijlstra A. Fuchs’ heterochromic cyclitis: review of the literature on the pathogenetic mechanisms. Br J Ophthalmol. 1994;78:307-312.
- de Groot-Mijnes JD, de Visser L, Rothova A, et al. Rubella virus is associated with fuchs heterochromic iridocyclitis. Am J Ophthalmol. 2006;141:212-214.
- Birnbaum AD, Tessler HH, Schultz KL, et al. Epidemiologic relationship between Fuchs heterochromic iridocyclitis and the United States rubella vaccination program. Am J Ophthalmol. 2007;144:424-428.
- Wakefield D, Chang JH. Epidemiology of uveitis. Int Ophthalmol Clin. Spring. 2005;45:1-13.
- Tandon M, Malhotra PP, Gupta V, Gupta A, Sharma A. Spectrum of Fuchs uveitic syndrome in a North Indian population. Ocul Immunol Inflamm. 2012 Dec;20(6):429-433.
- Tugal-Tutkun I, Güney-Tefekli E, Kamaci-Duman F, Corum I. A cross-sectional and longitudinal study of Fuchs uveitis syndrome in Turkish patients. Am J Ophthalmol. 2009 Oct;148(4):510-515.
- Liesegang TJ. Clinical features and prognosis in Fuchs’ uveitis syndrome. Arch Ophthalmol. 1982;100:1622-1626.
- Jones Fuchs' heterochromic uveitis: an update. Surv Ophthalmol. 1993 Jan-Feb;37(4):253-272. Review.
- Jones Glaucoma in Fuchs’ Heterochromic Uveitis: aetiology, management and outcome. Eye (Lond). 1991;5:662-667.
- Cokington CD, Han Bilateral nongranulomatous uveitis and a post streptococcal syndrome. Am J Ophthalmol. 1991; 112:595-596.
- Wirostko WJ, Connor TB, Wagner Recurrent post streptococcal uveitis. Arch Ophthalmol. 1999;117:1649-1650.
- Benjamin A, Tufail A, Holland GN. Uveitis as the only clinical manifestation of post streptococcal syndrome. Am J Ophthalmol. 1997;123:258-260.
- Holland GN. Recurrent anterior uveitis associated with streptococcal pharyngitis in a patient with a history of post streptococcal Am J Ophthalmol. 1999;127:345-347.
- Ur Rehman S, Anand S, Reddy A, et al. Poststreptococcal syndrome uveitis: a descriptive case series and literature Ophthalmology. 2006;113(4): 701-706.
- Birnbaum AD, Jiang Y, Vasaiwala R, Tessler HH, Goldstein DA. Bilateral simultaneous-onset nongranulomatous acute anterior uveitis: clinical presentation and etiology. Arch Ophthalmol. 2012 Nov;130(11):1389-1394.
- Amigo MC, Martı´nez-Lavı´n M, Reyes Acute rheumatic fever. Rheum Dis Clin North Am. 1993;19(2):333-350.
- Tubergen DG, Bleyer A, and RitcheyIn KA. The Leukemias. In: Kliegman R, Stanton B, Schor N, St.Geme J, Behrman R, eds. Nelson Textbook of Pediatrics. 19th ed. Elsevier Health Sciences, 2011: 1732-1739.
- Newman NM, Smith ME, Gay AJ. An unusual case of leukemia involving the eye: a clinico-pathological Surv Ophthalmol. 1972 Mar-Apr;16(5):316-321.
- Martin B. Infiltration of the iris in chronic lymphatic leukaemia. Br J Ophthalmol. 1968 Oct;52(10):781-785.
- Rowan PJ, Sloan JB. Iris and anterior chamber involvement in leukemia. Ann Ophthalmol. 1976 Sep;8(9):1081-1085.
- Zakka KA, Yee RD, Shorr N, Smith GS, Pettit TH, Foos Leukemic iris infiltration. Am J Ophthalmol. 1980 Feb;89(2):204-209.
- Wolintz AH, Goldstein JH, Seligman BR, Rosner F, Wesely AC, Lee SL. Secondary glaucoma in leukaemia. Ann Ophthalmol. 1971; 3: 1211-1213.
- Abramson DH, Schefler AC. Update on retinoblastoma. Retina. 2004 Dec;24:828-848.
- Stafford WR, Yanoff M, Parnell BL. Retinoblastomas initially misdiagnosed as primary ocular inflammations. Arch Ophthalmol. 1969 Dec;82(6):771-773.
- Croxatto JO, Fernadez MR, Malbran ES. Retinoblastoma masquerading as ocular inflammation. Ophthalmologica. 1986: 48-53,
- Hamel P, Budning AS, Heon E, et al. Focal Therapy in the Management of Retinoblastoma: When to Start and When to Stop. J AAPOS. 2000;4:334-337.
- Lin P, O’Brien JM. Frontiers in the Management of Retinoblastoma. Am J Ophthalmol. 2009; 148:142-148.
- Tahan SR, Pastel-Levy C, Bhan AK, Mihm MC Juvenile xanthogranuloma. Clinical and pathologic characterization. Arch Pathol Lab Med. 1989 Sep;113(9):1057-1061. PubMed PMID: 2505733.
- Lewis JR, Drummond GT, Mielke BW, Hassard DT, Astle Juvenile xanthogranuloma of the corneoscleral limbus. Can J Ophthalmol. 1990 Dec;25(7):351-354. PubMed PMID: 2128619.
- Wertz FD, Zimmerman LE, McKeown CA, Croxatto JO, Whitmore PV, LaPiana FG. Juvenile xanthogranuloma of the optic nerve, disc, retina, and choroid. Ophthalmology. 1982 Dec;89(12):1331-1335.
- DeBarge LR, Chan CC, Greenberg SC, McLean IW, Yannuzzi LA, Nussenblatt RB. Chorioretinal, iris, and ciliary body infiltration by juvenile xanthogranuloma masquerading as uveitis. Surv Ophthalmol. 1994 Jul-Aug;39(1):65-71.
- Schwartz LW, Rodrigues MM, Hallett Juvenile xanthogranuloma diagnosed by paracentesis. Am J Ophthalmol. 1974. Feb;77(2):243-246.
- Clements DB. Juvenile xanthogranuloma treated with local steroids. Br J Ophthalmol. 1966 Nov;50(11):663-665.
- Caplash S, Gangaputra S, Kesav N, Akanda M, Vitale S, Kodati S, Marques A, Sen HN. Usefulness of Routine Lyme Screening in Patients with Uveitis. Ophthalmology. 2019 Dec;126(12):1726-1728.
- MacLeod PM. Juvenile xanthogranuloma of the iris managed with superficial radiotherapy. Clin Radiol. 1986 May;37(3):295-296.
- Rosenbaum JT, Tammaro J, Robertson JE Uveitis precipitated by nonpenetrating ocular trauma. Am J Ophthalmol. 1991 Oct 15;112(4):392-395.
- Birnbaum AD, Oh FS, Chakrabarti A, Tessler HH, Goldstein DA. Clinical features and diagnostic evaluation of biopsy-proven ocular sarcoidosis. Arch Ophthalmol. 2011 Apr;129(4):409-413.
- Kaiser PK, Lowder CY, Sullivan P, et al. Chest computerized tomography in the evaluation of uveitis in elderly women. Am J Ophthalmol. 2002 Apr;133(4):499-505.
- Birnbaum AD, Fagan BM, Tessler HH, Goldstein DA. Risks of computerized tomography in the evaluation of chronic uveitis. Am J Ophthalmol. 2005 May;139(5):951-952.
- de Boer J, Wulffraat N, Rothova A. Visual loss in uveitis of childhood. Br J Ophthalmol 87:879-884,
- Rosenberg KD, Feuer WJ, Davis JL: Ocular complications of pediatric uveitis. Ophthalmology. 111:2229-2306,
- Edelsten C, Reddy MA, Stanford MR et al: Visual loss associated with pediatric uveitis in English primary and referral centers. Am J Ophthalmol. 135:676-680,
- Kotaniemi K, Kautiainen H, Karma A, Aho KL: Occurrence of uveitis in recently diagnosed juvenile chronic arthritis: A prospective Ophthalmology. 108:2071-2075, 2001.
- Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010 Jul;117(7):1436-1441.
- Lam LA, Lowder CY, Baerveldt G, et al. Surgical management of cataracts in children with juvenile rheumatoid arthritis-associated uveitis. Am J Ophthalmol. 2003 Jun; 135(6):772-778.
- Foster CS, Havrlikova K, Baltatzis S et al: Secondary glaucoma in patients with juvenile rheumatoid arthritis-associated iridocyclitis. Acta Ophthalmol Scand 78:576-579,
- Kafkala C, Hynes A, Choi J et al: Ahmed valve implantation for uncontrolled pediatric uveitic J AAPOS 9:336-340, 2005.
- Jain R, Ferrante P, Reddy GT, Lightman S. Clinical features and visual outcome of intermediate uveitis in children. Clin Experiment Ophthalmol. 2005;33(1):22-25.
- Lustig MJ, Cunningham ET Use of immunosuppressive agents in uveitis. Curr Opin Ophthalmol. 2003;14(6):399-412.
- Birnbaum AD, Jiang Y, Tessler HH, Goldstein DA. Elevation of intraocular pressure in patients with uveitis treated with topical difluprednate. Arch Ophthalmol. 2011 May;129(5):667-8.
- Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012 May;153(5):932-8.
- Sallam A, Comer RM, Chang JH, Grigg JR, Andrews R, McCluskey PJ, Lightman S. Short-term safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema in children. Arch Ophthalmol. 2008 Feb;126(2):200-5.
- Tugal-Tutkun I. Pediatric uveitis. J Ophthal Vision Res. 2011; 6:259-269.
- Kim SJ. Diagnosis and management of noninfectious pediatric uveitis. Int Ophthalmol Clin. 2011; 51:129-145.
- Taylor SR, Tomkins-Netzer O, Joshi L, et al. Dexamethasone implant in pediatric Ophthalmology. 2012; 119:2412-2412.e2.
- Patel CC, Mandava N, Oliver SC, et al. Treatment of intractable posterior uveitis in pediatric patients with the fluocinolone acetonide intravitreal implant (Retisert). Retina. 2012; 32:537-542.
- Mehta PJ, Alexander JL, Sen HN. Pediatric uveitis: new and future treatments. Curr Opin Ophthalmol. 2013 Sep;24(5):453-62.