Introduction
The mnemonic STUMPED is helpful for remembering the differential diagnosis for congenital corneal opacities: sclerocornea, tears in Descemet membrane (usually due to forceps trauma or congenital glaucoma), ulcers (infection), metabolic (eg, mucopolysaccharidosis), Peters anomaly, edema (eg, congenital hereditary endothelial dystrophy [CHED], posterior polymorphous dystrophy, congenital hereditary stromal dystrophy [CHSD], glaucoma), and dermoid. An alternative classification of corneal opacities is based on whether they are primary versus secondary, or congenital versus acquired (see Table 1).
|
Table 1. Differential diagnosis for congenital and acquired corneal opacities in the pediatric population.
|
|
Congenital Corneal Opacities
|
|
Localized/Non-Systemic Disease
|
Systemic/Metabolic Disorders
|
|
Congenital hereditary endothelial dystrophy
|
Mucopolysaccharidoses
|
|
Posterior polymorphous corneal dystrophy
|
Cystinosis
|
|
Congenital hereditary stromal dystrophy
|
Tyrosinemia
|
|
Peters anomaly
|
Schnyder crystalline dystrophy
|
|
Congenital anterior staphyloma
|
Fetal alcohol syndrome
|
|
Sclerocornea
|
|
|
Cornea plana
|
|
|
Cornea tumors
|
|
|
Epibulbar dermoid
|
|
|
|
|
Acquired Corneal Opacities
|
|
Traumatic
|
Non-Traumatic
|
|
Traumatic breaks in Descemet membrane
|
Keratoconus
|
|
Corneal blood staining
|
Posterior corneal depression
|
|
Corneal keloids
|
Keratoglobus
|
|
|
Congenital or infantile glaucoma
|
|
|
Corneal ulcers
|
|
|
Familial dysautonomia (Riley-Day syndrome)
|
|
|
Stevens-Johnson syndrome and toxic epidermal necrolysis
|
Congenital Corneal Opacities
Localized / Non-systemic disease
Congenital hereditary endothelial dystrophy
CHED is an uncommon corneal dystrophy. The cornea is diffusely and uniformly edematous because of a defect of the corneal endothelium and Descemet membrane. The edema involves both the stroma and the epithelium and is typically a bilateral process. The appearance of the cornea is similar to that in congenital glaucoma but without increased corneal diameter and elevated intraocular pressure. The hallmark of CHED is increased corneal thickness. CHED type I is the same entity as posterior polymorphous dystrophy (PPMD); both are autosomal dominant on the same locus at pericentromeric chromosome 20 and present in the first or second decade of life without nystagmus. CHED type II presents at birth with nystagmus and is autosomal recessive. Deafness and CHED are seen in Harboyan syndrome.
Reports in the literature of graft survival and outcomes from penetrating keratoplasty are good, as are more recent reports of success with Descemet stripping endothelial keratoplasty (DSEK).2-4
Posterior polymorphous dystrophy
PPMD, also known as posterior polymorphous corneal dystrophy (PPCD), presents at birth only rarely; it usually presents in the second or third decade of life. It is a disease of the endothelial cells. It presents with either an arrangement of vesicular lesions in isolation, in a band pattern, or in a more diffuse pattern. About half the time it is a combination. The endothelial cells that abut the lesions are typically pleomorphic. This irregular endothelium takes on epithelial characteristics with multi-layering seen on histopathologic sectioning and stains positively for cytokeratin.4 Peripheral anterior synechiae (PAS) are also a characteristic feature of PPMD; they can range from only being seen on gonioscopy to being extensive enough to be found during standard slit lamp examination. This is a particularly important finding to identify, as PAS can be a poor prognostic indicator for corneal transplant survival. These patients are also prone to both open-angle and closed-angle glaucoma. Iridocorneal endothelial (ICE) syndrome presents very similarly, with endothelial reduplication, corneal edema, glaucoma, corectopia, and PAS; however, ICE tends to be sporadic and unilateral.5
Congenital hereditary stromal dystrophy
CHSD is a very rare congenital stationary opacification of the cornea, transmitted in an autosomal dominant manner. A flaky or feathery clouding of the stroma, which is of normal thickness, is covered by a smooth, normal epithelium. These features are in contrast with those of CHED, which exhibits a thickened stroma and epithelial edema.6
Peters anomaly7-8
Peters anomaly, also known as iridocorneal adhesions or keratolenticular adhesions, is a posterior corneal defect with an overlying stromal opacity, often accompanied by adherent iris strands (Peters anomaly type 1). The size and density of the opacity can range from a mild to dense central leukoma. The strands from the iris to the borders of this defect vary in number and density. In severe cases, the central leukoma may be vascularized and protrude above the level of the cornea. The stromal opacity may decrease with time. Lysis of adherent iris strands has been reported to improve corneal clarity. A more severe variety includes adherence of the lens to the cornea at the site of the central defect (Peters anomaly type 2). Peters anomaly can be caused by many different diseases, including genetic conditions (eg, Axenfeld-Rieger syndrome) and non-genetic conditions (eg, congenital rubella). Unilateral cases are usually isolated. Bilateral cases are often associated with systemic disorders and warrant a complete genetic workup. A syndrome called microphthalmia with linear skin defects (MLS) includes microphthalmos, reddish linear skin lesions, and life-threatening cardiac arrhythmias. Peters-plus syndrome is bilateral Peters anomaly associated with congenital brain defects, heart defects, and craniofacial anomalies.
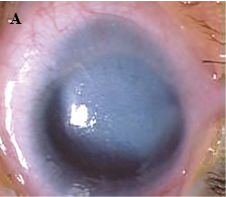
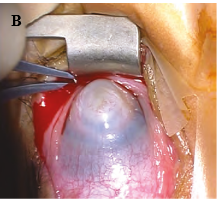
Figure 1. A, Corneal opacity secondary to iridocorneal adhesion (Peters anomaly type 1). B, Corneal opacity secondary to keratolenticular adhesion (Peters anomaly type 2). (Courtesy of Ken K. Nischal, MD.)
Congenital Anterior Staphyloma5,19
Congenital anterior staphyloma is a rare form of anterior segment dysgenesis that shares similarities with Peters anomaly. It is characterized by an ectatic protrusion of a central opacified cornea lined by uveal tissue. The protrusion extends beyond the plane of the eyelid margins and it can be unilateral or bilateral. The corneal stroma is typically thickened, vascularized, and fibrosed, with variable alterations in the Bowman membrane and the epithelium, and an absence of Descemet and endothelium. Heroic measures, including corneal transplant, can be attempted, but this invariably leads to very poor vision or blindness as a result of the significant disarray of the anterior segment structures.
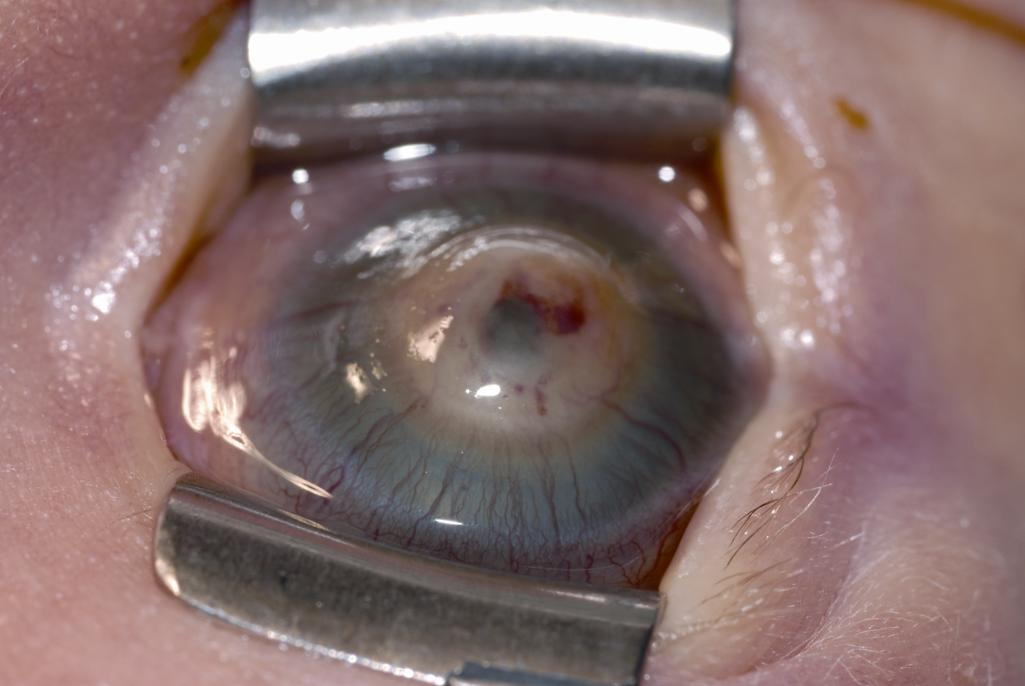
Figure 2. Congenital anterior staphyloma. See the central corneal protrusion with posterior corneal pigmentation from uveal tissue. (© 2015 American Academy of Ophthalmology.)
Sclerocornea
Sclerocornea is a congenital disorder in which the cornea is opaque and resembles the sclera, making the limbus indistinct. The central cornea is clearer than the periphery in nearly all cases, in contradistinction to Peters anomaly, in which the periphery is generally clearer. Severe cases show no increased corneal curvature and no apparent scleral sulcus. Sclerocornea is often associated with other ocular or systemic abnormalities. Be particularly mindful that some use the term sclerocornea as more of an examination finding than a disease entity of its own. It should not be used to describe total corneal opacification, and it always requires further investigation; Anterior segment imaging (ultrasound biomicroscopy; UBM) is important for detecting other abnormalities and guiding treatment.
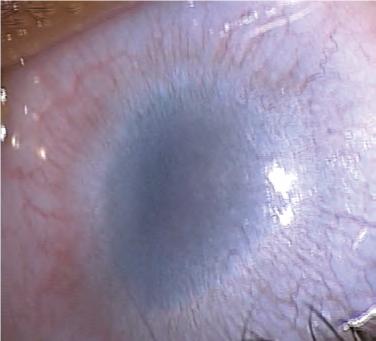
Figure 3. Sclerocornea with cornea plana. (Courtesy of Ken K. Nischal, MD. © 2015 American Academy of Ophthalmology.)
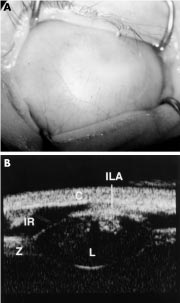
Figure 4. A, This image shows complete corneal opacification thought clinically to be sclerocornea. B, The UBM of the same eye shows keratolenticular adhesion (ILA), aniridia with only an iris stump detected (IR), a small lens (L), and thickened-looking zonules (Z). The cornea is also seen (C). This case from Ken Nischal, MD, demonstrates the profound importance of a complete anterior segment examination with UBM to make an accurate diagnosis and appropriate surgical plan, if any. The post-UBM diagnosis changed to Peters Anomaly. (Reproduced from the British Journal of Ophthalmology, Nischal KK, Naor J, Jay V, MacKeen LD, Rootman DS. ©2002; 86:62-69, with permission from BMJ Publishing Group Ltd.)
Cornea Plana
Cornea plana is a rare, bilateral, often autosomal recessive condition that features flat corneas, peripheral scleralization of the cornea, and a shallow anterior chamber. Patients are often hyperopic, and glaucoma may develop secondary to angle closure due to the shallow anterior chamber. Refractive correction and glaucoma management are the mainstays of treatment.
Cornea Tumors
Tumors of the cornea are extremely rare in children, but squamous cell carcinomas have been reported in cases of xeroderma pigmentosum.
Epibulbar dermoid
An epibulbar (limbal) dermoid is a choristoma composed of fibrofatty tissue covered by keratinized epithelium. It is present from birth, and little if any postnatal growth occurs. Dermoids may contain hair follicles, sebaceous glands, or sweat glands. They can be up to 10 mm in diameter and usually straddle the limbus. They may extend into the corneal stroma and adjacent sclera but seldom occupy the full thickness of either cornea or sclera. Most epibulbar dermoids are on the inferior temporal limbus. Many produce a lipoid infiltration of the corneal stroma at their leading edge. They are sometimes continuous with subconjunctival dermolipomas that involve the lateral quadrant of the eye.
Epibulbar dermoids can produce anisometropic astigmatism with secondary amblyopia. Large epibulbar dermoids can cover the visual axis. Removal of epibulbar dermoids may be indicated if they cause ocular irritation or amblyopia. Surgical excision may result in scarring and astigmatism, which can also lead to amblyopia. Although excision will not eliminate the preexisting astigmatism, surgery may be useful for treating very elevated lesions. Tumor removal involves excising the episcleral portion flush with the plane of surrounding tissue. In general, the surgeon need not remove underlying clear corneal tissue, mobilize surrounding tissue, or apply a patch graft over the resulting surface defect; however, because some lesions extend into the anterior chamber, tissue should be available in the event that a patch graft is required. Cornea and conjunctiva heal within a few days to several weeks, generally with some scarring and imperfect corneal transparency; nevertheless, the appearance can be improved considerably.
Epibulbar dermoids are often seen with Goldenhar syndrome (oculoauriculovertebral spectrum). These patients may have a variety of other anomalies, including ear deformities, maxillary or mandibular hypoplasia, vertebral deformities, eyelid colobomas, and Duane retraction syndrome.
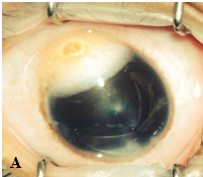
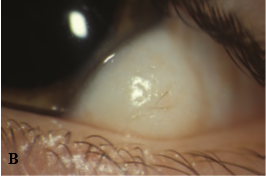
Figure 5. A, Large epibulbar dermoid extending onto the cornea. B, Epibulbar dermoid with hair growing in the center. (Courtesy of Ken K. Nischal, MD. © 2015 American Academy of Ophthalmology.)
Systemic/ Metabolic Disorders
Mucopolysaccharidoses
Corneal haze may be present in early life in 3 lysosomal disorders: by age 6 months in mucopolysaccharidosis I H (Hurler syndrome); by age 12–24 months in mucopolysaccharidosis I S (Scheie syndrome); and as early as age 6 weeks in mucopolysaccharidosis IV (Morquio syndrome). Treatment options for significant opacities include penetrating keratoplasty and DALK. Metabolic diseases cloud the cornea via accumulation of a pathway product. If the product is produced in the cornea, the clouding may be found throughout the cornea. If the level of the product is elevated in the blood, the peripheral cornea alone may be involved. Diseases that affect the cornea include the mucopolysaccharidoses (MPS; types I H, I S, I H/S, II, IV, VI, and VII), cystinosis, and Wilson disease.
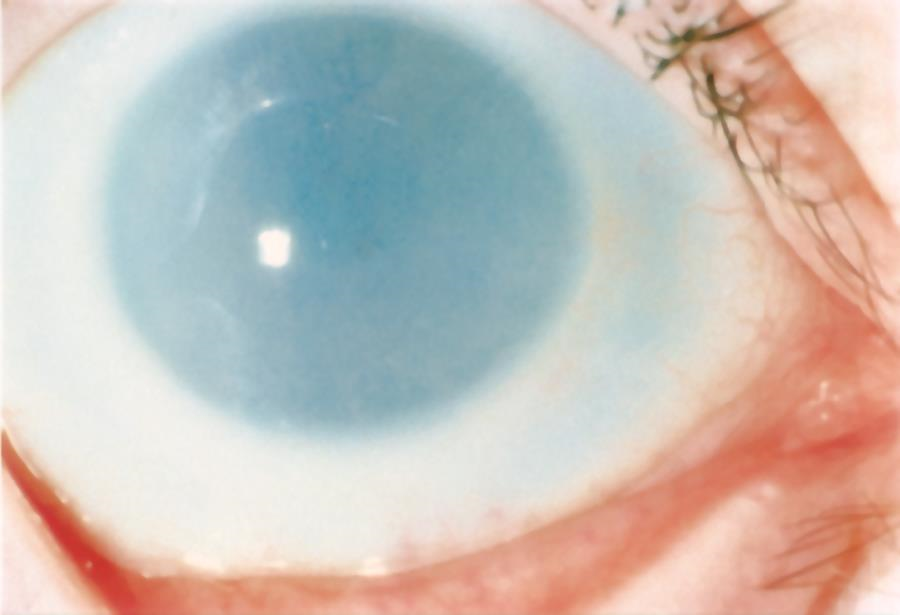
Figure 6. Corneal clouding in MPS I (hurler) syndrome. (© 2015 American Academy of Ophthalmology.)
Cystinosis5
Cystinosis is a rare metabolic disease characterized by elevated levels of cystine within the cell. Cystine crystals are deposited in various places throughout the body. The major presenting symptoms of the infantile form of cystinosis are failure to thrive, rickets, and progressive renal failure, collectively resulting in Fanconi syndrome. The ocular findings of cystinosis are pathognomonic. Iridescent elongated corneal crystals appear at approximately age 1 year, first in the peripheral part of the cornea and the anterior part of the stroma. Severe photophobia can make slit-lamp examination difficult. These crystals are also present in the uvea and on the surface of the iris. As survival has improved, reports of angle-closure glaucoma secondary to crystal deposition in the ciliary body have increased. Oral cysteamine has been shown to alleviate the systemic problems but not the corneal crystal deposition. Topical cysteamine can reduce crystal deposition in the cornea. However, the medication may be difficult to obtain and has an unpleasant odor, and treatment is complicated by the need for frequent application.
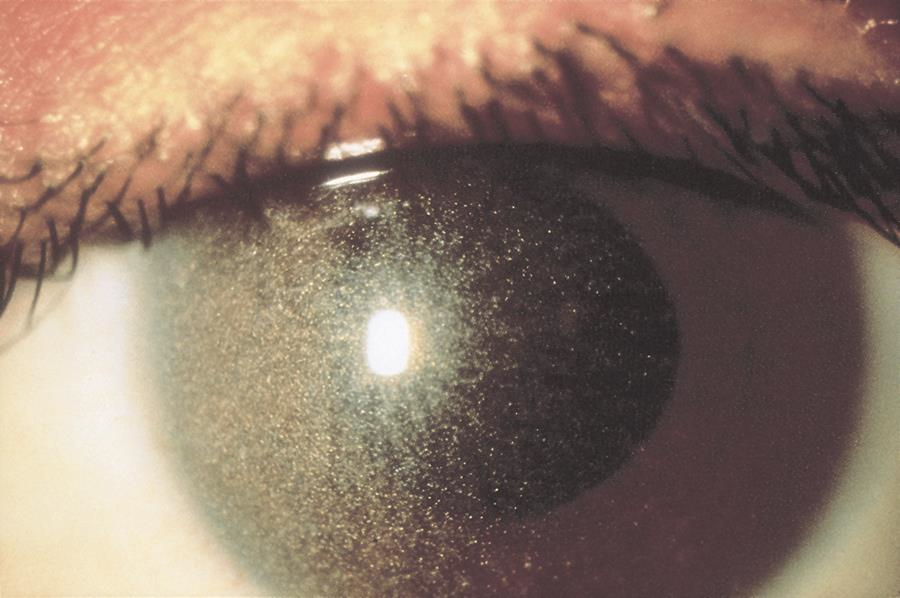
Figure 7. Crystalline keratopathy from cystinosis. (© 2015 American Academy of Ophthalmology.)
Tyrosinemia
Children with tyrosinemia often present with photophobia, pseudodendritic ulcers on the cornea, and ulceration on the palms and soles. Systemic problems include liver and kidney dysfunction.
Schnyder crystalline dystrophy5,9-11
Schnyder crystalline dystrophy is a rare autosomal dominant condition caused by mutations in the UBIAD1 gene and is thought to be a local disorder of corneal lipid metabolism. It is associated with hypercholesterolemia but not directly associated with any of the primary hyperlipidemias. Varying patterns of bilateral central corneal deposits occur primarily in the anterior stromal layers early in the course, but become deeper over time and associated with arcus seniles or arcus lipoides after age 23. It tends to be nonprogressive after childhood, and rarely affects vision enough to warrant surgical intervention early in its course. Over time, however, visual acuity can deteriorate and complications from decreased corneal sensation can follow, potentially requiring surgical intervention with lamellar or penetrating keratoplasty.
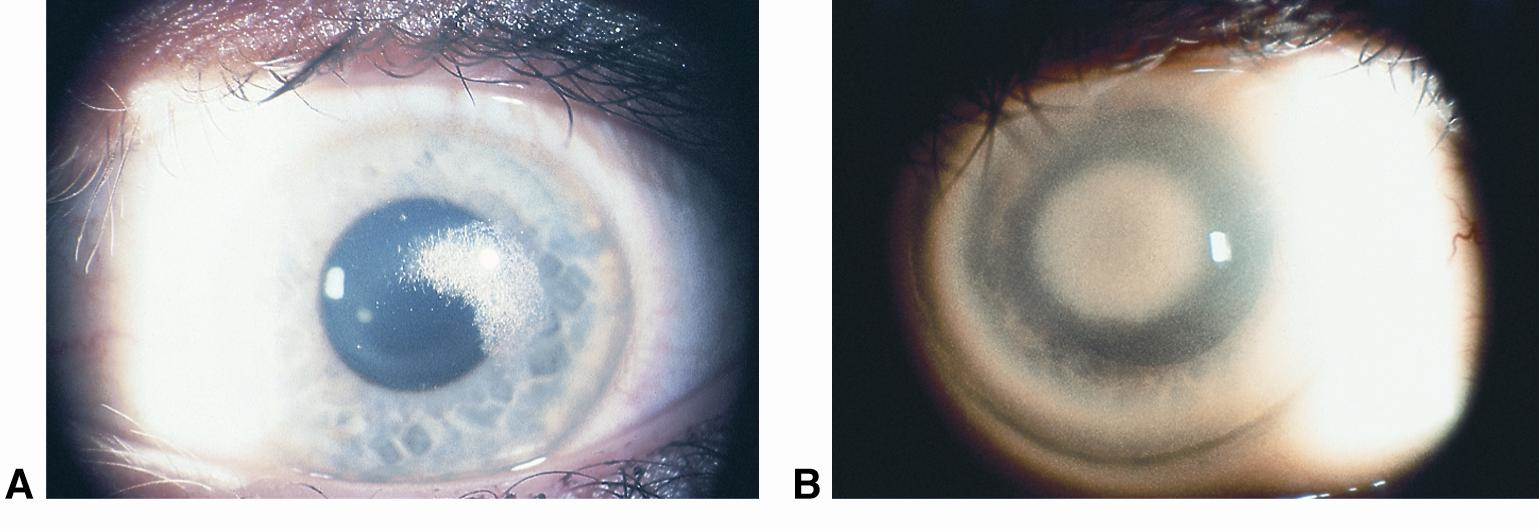
Figure 8. Schnyder corneal dystrophy with (A) central subepithelial crystalline deposition and (B) central panstromal corneal opacity and arcus lipoides. No crystals are present. (Courtesy of Jayne S. Weiss, MD. © 2015 American Academy of Ophthalmology.)
Fetal alcohol syndrome5,12-14
Fetal alcohol syndrome (FAS) is a craniofacial condition caused by in utero exposure to ethanol. It is characterized by prenatal and postnatal growth retardation, central nervous system and craniofacial abnormalities, and intellectual disability. The classic ocular features of FAS are short palpebral fissures, telecanthus, epicanthus, ptosis, microphthalmos, and esotropia. Anterior segment dysgenesis, optic nerve hypoplasia, and high refractive errors have been reported. Fifty percent of children with this underdiagnosed syndrome have some form of visual impairment. The patients can be born with cloudy corneas or develop them soon after birth. Pathologic studies have confirmed abnormalities in Descemet membrane and endothelium that results in corneal edema. Infants with FAS can have iridocorneal adhesions (Peters), Axenfeld-Rieger syndrome, or diffuse corneal edema.
Acquired Corneal Opacities
Traumatic
Traumatic breaks in Descemet membrane
Injuries to Descemet membrane may be caused by forceps trauma to the eye during delivery. These are usually vertical and linear, whereas the Haab striae seen in congenital glaucoma are curvilinear. Rupture of Descemet membrane leads to stromal and sometimes epithelial edema. Other signs of trauma are frequently apparent on the child’s head. In most cases, the stromal and epithelial edema regresses, but the edges of the broken Descemet membrane persist and can be seen as ridges protruding slightly from the posterior corneal surface. Amblyopia may result from the corneal opacity. Anisometropic astigmatism induced by the trauma can cause severe amblyopia even if the corneal haze resolves quickly. Follow-up examinations are indicated, as optical correction and patching may be required.
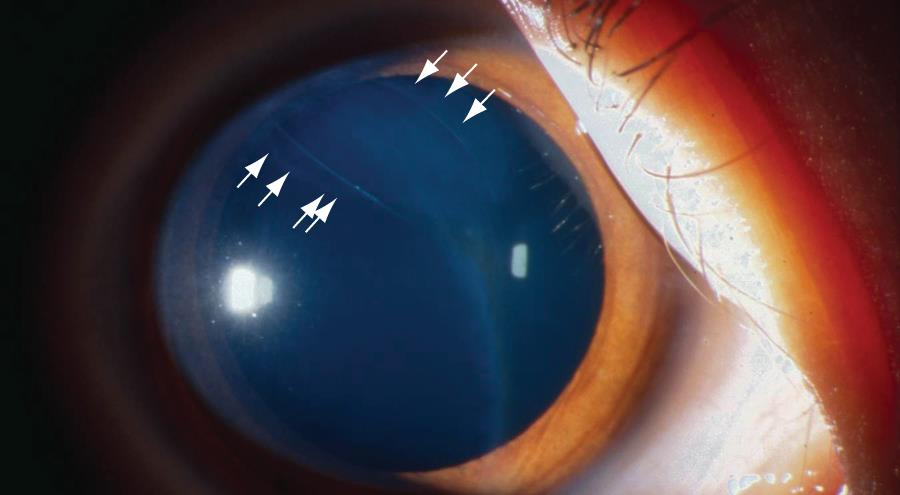
Figure 9. Break in Descemet membrane (arrows) after forceps injury. (Courtesy of C. Gail Summers, MD. © 2015 American Academy of Ophthalmology.)
Trauma from amniocentesis is a rare cause of unilateral corneal opacification in a newborn. Other corneal trauma leading to scarring has the same anisometropic/amblyopic concerns as the above. Corneal blood staining that typically results from a hyphema and elevated intraocular pressure can be particularly problematic for children in the amblyogenic phase, as the densely packed yellow pigmentation in the stroma classically clears from the limbus in a centripetal fashion. If large enough, this could theoretically warrant the need for a penetrating keratoplasty, as the natural time-course for healing can range from months to over a year. Aside from preventing the development of corneal blood staining in children by intervening surgically as indicated (intraocular pressure management and/or clearing of anterior chamber blood clots or hyphemas), there are no therapies known to otherwise hasten the clearing of the stroma.
Corneal keloids15-17
Corneal keloids are fibrous proliferations that result from a vigorous wound-healing response to injury, but have also been reported as congenital lesions. They appear as white, protuberant, glistening masses, and can be distinguished from corneal dermoids and nodules of Salzmann degeneration by biopsy. They can replace the entire cornea and even invade into the anterior chamber. When visually significant, removal is necessary by means of superficial or anterior lamellar keratectomy, deep anterior lamellar keratoplasty (DALK), penetrating keratoplasty, and sclerokeratoplasty. Unpublished testimonial advice from colleagues within the cornea community suggests avoiding the use of excimer laser as the modality for keratectomy, as this tends to lead to recurrence. Complications other than recurrence include persistent epithelial defects and recurrent corneal erosions, with one case report of success with amniotic membrane transplant.
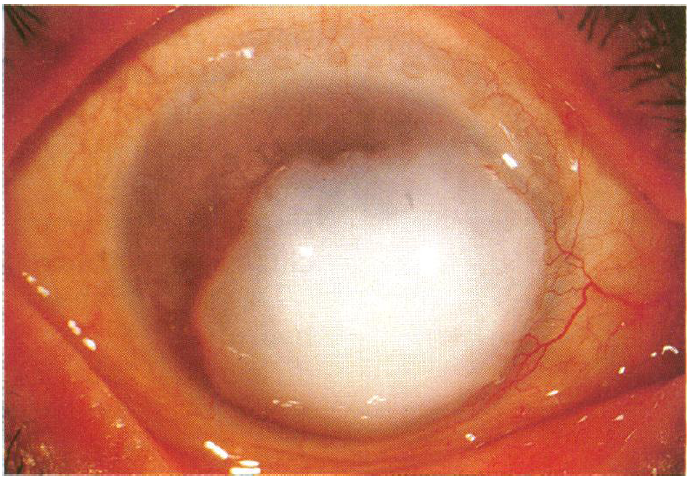
Figure 10. A large corneal keloid that was excised without complication by superficial keratectomy. (Reproduced from the British Journal of Ophthalmology, Risco JM, Huaman A, Antonios SR. ©1994; 78: 568-571, with permission from BMJ Publishing Group Ltd.)
Non-traumatic
Keratoconus
In keratoconus, the central or paracentral cornea bulges and progressively thins such that the cornea takes on the shape of a cone. Keratoconus may present and progress during the adolescent years. It can occur with Down syndrome, atopic diseases, and chronic eye rubbing. Keratoconus may be familial. Unlike in keratoglobus, iron lines, stress lines (Vogt striae), and apical scarring are often seen. Tears in Descemet membrane can cause acute corneal edema (hydrops) in patients with keratoconus.
Posterior corneal depression
Posterior corneal depression (central posterior keratoconus), a discrete posterior corneal indentation, produces an abnormal red reflex during examination with a retinoscope or direct ophthalmoscope. A highly convex posterior corneal surface is visible with slit-lamp examination. Pigment deposits sometimes appear on the border of the posterior defect. The anterior curvature of the cornea is normal.
This defect usually causes irregular astigmatism and can result in amblyopia. If refractive correction is not successful, Descemet stripping endothelial keratoplasty (DSEK) can be considered.
Keratoglobus
Keratoglobus is a very rare autosomal recessive, bilateral noninflammatory condition present at birth. It may occur in Ehlers-Danlos syndrome type VI. The corneal curvature is steep, with thinning of the cornea in the periphery and a very deep anterior chamber. Spontaneous breaks in Descemet membrane may produce acute corneal edema, and the cornea is easily ruptured by minor blunt trauma. Patients with keratoglobus should routinely wear protective lenses. Scleral contact lenses may be helpful.
Congenital or infantile glaucoma
Glaucoma in an infant can cause the cornea to become edematous, cloudy, and enlarged. The details of congenital glaucoma are beyond the scope of this chapter.
Corneal ulcers5
Corneal ulcers that are present at or develop around birth are rare and may be caused by herpes simplex (HSV) keratitis, bacterial keratitis, or neurotrophic keratitis. Identifying HSV keratitis in the early perinatal period is critical, as ocular findings may precede the systemic infection that results in poor morbidity and mortality. Congenital rubella is an extremely rare condition in the developed world due to the success of vaccination programs, but should remain on the differential. Congenital rubella is acquired during the first trimester of gestation, and corneal opacity may result from an endotheliitis, elevated intraocular pressure, or keratolenticular adhesions (Peters anomaly). The former two causes are self-limited and resolve within several weeks. Surgical intervention is warranted if the opacities do not clear, or if there are structuring abnormalities such as those seen in Peters.
Familial dysautonomia
Familial dysautonomia (Riley-Day syndrome), a complex autosomal recessive condition, occurs largely in children of Eastern European Jewish (Ashkenazi) descent. It is characterized by autonomic dysfunction, relative insensitivity to pain, temperature instability, and absence of the fungiform papillae of the tongue. Exposure keratitis and corneal ulcers with secondary opacification are frequent problems because of abnormal lacrimation and decreased corneal sensitivity. Failure to respond with a wheal and flare to intradermal injection of 1:1000 histamine solution is characteristic of this condition. Treatment includes artificial tears and tarsorrhaphy. Most cases of familial dysautonomia are caused by a mutation in the IKBKAP gene.
Stevens-Johnson syndrome and toxic epidermal necrolysis18
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, life-threatening conditions that represent different intensities of an acute inflammatory systemic disease affecting skin and mucous membranes. In the pediatric population, the male-to-female ratio is 2:1. The mortality rate in the pediatric population, at 1%, is much lower than that of adults, although the morbidity is 45% and the recurrence rate is 18%. The most common etiologies of SJS and TEN in children are medications (usually anticonvulsants and sulfonamides) and infections (usually Mycoplasma species or herpes simplex virus). The pathogenesis of SJS and TEN is unknown. Systemic manifestations range from mild to severe. A prodrome of fever, malaise, and upper respiratory tract infection is followed by bullous mucosal and skin lesions. These lesions rupture, ulcerate, and become covered by gray-white membranes and a hemorrhagic crust. Ocular involvement, which occurs in as many as 50% of patients, varies from mild mucopurulent conjunctivitis to severe perforating corneal ulcers. Ocular involvement in SJS and TEN begins with edema, erythema, and crusting of the eyelids. The palpebral conjunctiva becomes hyperemic, and distinct vesicles or bullae may occur. In many instances, epithelial defects or ulcers involving the tarsus and fornices develop. In severe cases, membranous or pseudomembranous conjunctivitis may occur and lead to symblepharon formation. Superinfection, most commonly with Staphylococcus species, may develop.
Late ocular complications, possibly accompanied by a decrease in vision, occur in approximately 27% of pediatric patients. These complications include anomalies of eyelid position (ectropion and entropion), dry eye disease, trichiasis, chronic conjunctivitis, corneal defects, corneal vascularization, and symblepharon.
SJS and TEN are diagnosed based on clinical presentation and skin biopsy. Initial management includes treatment of any underlying infection and discontinuation of any inciting drug. Systemic therapy with corticosteroids or intravenous immunoglobulin is controversial. A full discussion of systemic treatment is beyond the scope of this article. A dermatologist and a specialist in pediatric infectious diseases should be consulted. Early intervention is important in preventing the late ocular complications of SJS and TEN. Ocular lubrication with artificial tears and ointments (preferably preservative free) should be applied frequently. Associated microbial infections should be treated. Sweeping of the fornices to lyse adhesions may be performed, although some ophthalmologists believe that doing so may stimulate inflammation and cause further scarring. In severe cases, a symblepharon ring may be useful in cooperative patients. In patients with significant ocular disease, amniotic membrane grafting should be considered early to decrease the risk of late ocular complications.
References
- Raab EL, Aaby A, Bloom JN, et al. Diseases of the Cornea, Anterior Segment, and Iris. In: Basic and Clinical Science Course, Section 6. Pediatric Ophthalmology and Strabismus. San Francisco: American Academy of Ophthalmology; 2014-2015: 241-262.
- Javadi MA, Baradaran-Rafii AR, Zamani M, et al. Penetrating keratoplasty in young children with congenital hereditary endothelial dystrophy. Cornea. 2003; 22:420-423.
- Schaumberg DA, Moyes AL, Gomes JA, Dana MR. Corneal transplantation in young children with congenital hereditary endothelial dystrophy. Multicenter Pediatric Keratoplasty Study. Am J Ophthalmol. 1999; 127:373-378.
- Goshe JM, Li JY, Terry MA. Successful Descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy in a pediatric patient. Int Ophthalmol. 2012; 32:61-66.
- Krachmer JH, Mannis MJ, Holland EJ. Congenital Corneal Opacities: Diagnosis and Management. In: Cornea: Fundamentals, Diagnosis and Management. 3rd Mosby Elsevier. 2011; 239-265.
- Weiss JS, Møller HU, Lisch W, et al. The IC3D classification of the corneal dystrophies. 2008; 27(Suppl 2):S1-S83.
- Bhandari R, Ferri S, Whittaker B, Liu M, Lazzaro DR. Peters anomaly: review of the literature. 2011; 30(8):939-944.
- Mataftsi A, Islam L, Kelberman D, Sowden JC, Nischal KK. Chromosome abnormalities and the genetics of congenital corneal opacification. Mol Vis. 2011; 17:1624-1640.
- Bron AJ, Williams HP, Carruthers ME: Hereditary crystalline stromal dystrophy of Schnyder. I. Clinical features of a family with hyperlipoproteinemia. Br J Ophthalmol. 1972; 56:383-399.
- Luxenberg M. Hereditary crystalline dystrophy of the cornea. Am J Ophthalmol. 1967: 63:507-511.
- Ehlers N, Mathiessen M: Hereditary crystalline corneal dystrophy of Schnyder. Acta Ophthalmol (Copenh). 1973; 51:316-324.
- Edward DP, Li J, Sawaguchi S, Sugar J, Yue BY, Tso MO. Diffuse corneal clouding in siblings with fetal alcohol syndrome. Am J Ophthalmol. 1993; 115 (4):484-493.
- Carones F, Brancato R, Venturi E, Bianchi S, Magni R. Corneal endothelial anomalies in the fetal alcohol syndrome. Arch Ophthalmol. 1992; 110 (8):1128-1131.
- Edward DP, Li J, Sawaguchi S, Sugar J, Yue BY, Tso MO. Diffuse corneal clouding in siblings with fetal alcohol syndrome. Am J Ophthalmol. 1993; 115:484-493.
- Vanathi M, Sen S, Panda A, Dada T, Behera G, Khokhar S. Unilateral congenital corneal keloid with anterior segment mesenchymal dysgenesis and subluxated lens: case report and review of literature. Cornea. 2007; 26:111-113.
- Rao SK, Fan DS, Pang CP, et al. Bilateral congenital corneal keloids and anterior segment mesenchymal dysgenesis in a case of Rubinstein–Taybi syndrome. Cornea 2002; 21:126-130.
- Chawla B, Agarwal A, Kashyap S, Tandon R. Diagnosis and management of corneal keloid. Clin Experiment Ophthalmol. 2007; 35:855-857. doi: 10.1111/j.1442-9071.2007.01608.x.
- Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: a review of 10 consecutive cases. 2011;118(5):908-914.
- Gupta DK, Mohan H, Sen DK. Congenital anterior staphyloma. Indian J Ophthalmol. 1969;17:64-66.