Definition (Disease Overview)
There are 2 types of retinal hemangiomas, based on clinical and histological characteristics: cavernous hemangioma1,2 and capillary hemangioma.3 Basically, a cavernous hemangioma is composed of clumps of dark intraretinal aneurysms and has a cluster-of-grapes aspect, while a capillary hemangioma is composed of tortuous, large-diameter capillaries lined by normal endothelium separated by polygonal interstitial stromal cells and usually have a “light bulb” appearance.2
Although both represent vascular hamartomas of the retina and may be associated with central nervous system and cutaneous changes,1,4 they each have a completely different natural history and prognosis that will be discussed herein.
Retinal Cavernous Hemangioma
Disease overview
Cavernous hemangiomas may be sporadic or syndromic.5 When syndromic, the inheritance is not well established, although some reports mention its occurrence with cerebral hemangiomas related to a splice mutation in CCM1/KRIT.4,6 Since the association of cerebral and cutaneous hemangiomas is not consistent, the cerebral cavernous malformation syndromes were not included in the neuro-oculo-cutaneous (phakomatoses) syndromes.5
Histopathologic analysis demonstrates sessile tumors consisting of thin-walled channels with surface gliosis and non-fenestrated endothelium. This may explain the lack of leakage of lipids and proteins to the interstitial retina.7
Incidence and statistics
The tumors are believed to be congenital and lesions may be seen at any age, but the average age is 23 years. They are more common in females (3:2 female:male). Most patients have only a solitary lesion affecting one eye, however multiple lesions may occur in one eye or sometimes in both eyes.2
Clinical guidelines
Retinal cavernous hemangiomas are usually asymptomatic and may be symptomatic in rare situations such as: 1) macular location of the hemangioma; 2) macular fibrosis or epiretinal membrane and 3) vitreous hemorrhage. Very rarely, retinal cavernous hemangiomas may lead to hyphemas in children.8
Retinal lesions have the appearance of grape-like clusters of dilated vascular sacs in the inner retinal layers without pronounced alteration in the adjacent arterioles, as well as venules with variable fibrosis on their surface (Figure 1). The size and locations of the hemangiomas are variable and may include the optic nerve. The tumors ordinarily do not progress and may undergo spontaneous regression. Vitreous hemorrhages are rare and usually self-limiting.
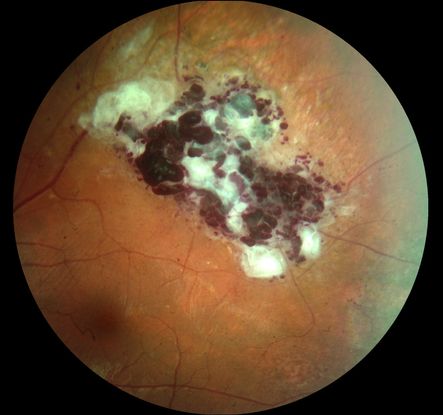
Figure 1. Color fundus pictures of the temporal superior equatorial region showing a retinal cavernous hemangioma with a typical cluster of grapes appearance and superficial gliosis. There is also a fine striae of the internal limiting membrane from the tumor to the foveal region.
Although retinal cavernous hemangiomas have a unique appearance on fundus examination, this lesion sometimes needs to be differentiated from retinal vasoproliferative tumors (usually at the periphery and associated with more fibrotic tissue and without the cluster of grapes pattern), retinal capillary hemangioma, racemose hemangioma (larger retinal vessels are dilated) and Coats Disease (pronounced exudation and retinal detachment). Retinal cavernous hemangiomas do not have feeder vessels and they are not associated with hard exudates.
Fluorescein angiography
Fluorescein angiography demonstrates that retinal cavernous hemangiomas are relatively isolated from the retinal circulation.2 There is slow filling of the sacular lesions and progressive hyperfluorescence due to staining of plasma contents within the lesions. There is no leakage, which is much different from capillary hemangiomas where there is rapid hyperfluorescence that progresses secondary to leakage from the fenestrated endothelium.
Systemic features
Despite the benign and usually asymptomatic course of retinal cavernous hemangiomas, cerebral cavernous hemangiomas may lead to important complications such as: seizures, intracranial hemorrhages and death. For this reason, neuroimaging is obligatory for patients with retinal cavernous hemangiomas.
Treatment
As the majority of cavernous hemangiomas of the retina remain stable over time, most can simply be followed with periodic observation. Some tumors may undergo spontaneous thrombosis and subsequently develop increased surface gliosis. A small number of reported cases have been associated with self-limiting vitreous hemorrhage. No effective treatment has been determined for these tumors; however, the use of laser photocoagulation has been reported in a few cases.5
Latest Developments
Imaging
Optical coherence tomography demonstrates an elevated intraretinal mass with superimposed sacular clusters and an overlying epiretinal membrane spanning the exterior of the lesion.9
Retinal Capillary Hemangioma
Disease Overview
Retinal capillary hemangioma is a benign retinal hamartoma that may be associated with von Hippel-Lindau (VHL) disease. Eugen Von Hippel was a German pathologist who initially described the lesion as angiomatosis retinae in 1904.10 Swedish pathologist Arvid Lindau suggested that the hemangiomas could occur on the cerebellum and retina.11 In 1964 the term von Hippel-Lindau was used to unify both diseases after recognition of their overlapping characteristics.12
The tumor is composed of a proliferation of endothelial and stromal glial cells. Immunohistochemical and immunocytochemical experiments have suggested that stromal cells are astrocytic, vasoformative stem cells and neuroectodermal in origin.13,14
The hemangioma may occur in 2 distinct forms, the peripheral lesion (von Hippel Tumor) or the juxtapapillary tumor. According to its morphology, it may be endophytic (inner retina), exophytic (outer retina) or sessile.
Endophytic tumors grow on the surface of the nerve or retina, protrude into the vitreous cavity, and are easily identifiable. Exophytic growth forms are nodular, orange-colored lesions that grow in the outer layers of the retina, usually close to the optic disc head. Tumors with a sessile growth pattern are relatively flat, gray or orange in color, and develop in the middle layers of the retina.15
Association with von Hippel Lindau (VHL) disease
The inheritance of VHL is autosomal dominant with high penetrance. The penetrance of VHL disease is age-dependent, achieving full penetrance by 65 years of age. Germline mutations within the VHL gene have been identified in up to 100% of affected families.16
The VHL gene is a classic tumor suppressor gene located on the short arm of chromosome 3 (3p25–p26) and bi-allelic inactivation by two genetic alterations must occur for tumor development. This is called a 2-hit model: 2 alleles must be inactivated. The first hit or inactivation is an inherited germline mutation and is present in one allele in all cells of the body. The second hit is a somatic DNA alteration, which is acquired during the patient’s lifetime and is present only in the tumor tissue. Several theories have been proposed to explain this second mutation, including age and DNA methylation. 17,18,19
Consequently, the tumors of these patients do not express the VHL protein, or may express a not functional mutated form. This protein is essential for hypoxia inducible factor (HIF) 1α and 2α ubiquitination and consequent degradation. In other words, the deficiency of VHL protein leads to an abnormal accumulation of hypoxia inducible factor and the consequent translocation of this factor into the nucleus of the cell, where it stimulates genes responsible for the production of proangiogenic factors, including VEGF, which are responsible for the vascular changes (vasodilation, vascular shunts, exudation and edema) and abnormal tissue proliferation of endothelial and stromal cells (hemangiomas).20
Incidence and statistics
The incidence of isolated retinal capillary hemangiomas is unknown, however the birth incidence of VHL is approximately one in 36,000. The tumor is usually detected by the second or third decade of life, but is has been reported from birth to 80 years of age. The median age at presentation for VHL disease is usually lower than the sporadic form: 17.6 years and 30.8 years, respectively. The tumor is more frequent in whites and there is no sex predilection. Approximately one-third of patients have multiple retinal capillary hemangiomas and up to half the patients have bilateral involvement.
Clinical Guidelines
Capillary hemangiomas are typically red or pink tumors originated from the inner retinal layers and they protrude into the vitreous (Endophytic tumors). When located peripherally to the optic nerve, these tumors are usually associated with arteriovenous shunting between a dilated tortuous artery and a draining vein (Figure 2). The tumors may also originate from the outer retinal layers, and these are classified as exophytic. These lesions are usually located in the juxtapapillary area and not associated with arteriovenous shunts. They are also usually red/pink intraretinal lesions and may be associated with edema, (simulating optic nerve edema) and juxtapapillary choroidal neovascularization.
Loss of vision secondary to edema and accumulation of hard exudates in the macula may occur in either peripheral or juxtapapillary lesions.2
Fluorescein Angiography is very useful to show progressive hyperfluorescence of juxtapapillary intraretinal lesions and to differentiate it from optic nerve edema and choroidal neovascularization. It is also useful to detect arteriovenous shunting in peripheral endophytic tumors, and to detect very small lesions (Figure 2: middle left).
Optical Coherence Tomography (OCT) is helpful to monitor the amount of intraretinal and subretinal fluid in cases of macular edema,21 and is also helpful to detect small retinal capillary hemangiomas. OCT is also useful to demonstrate the retinal layers affected by the lesion to help in classifying the lesion as endophytic versus exophytic (Figure 2: bottom).
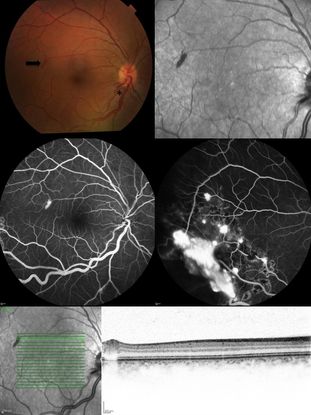
Figure 2. Top Left: Color fundus picture showing a discrete “rod-shaped” red lesion of a retinal capillary hemangioma temporal to the macula (arrow). There is also enlargement of the temporal inferior arteriole and venule close to the optic disc (asterisk). Top Right: Infrared reflectance picture of the posterior pole highlighting the lesion temporal to the fovea. Middle: Fluorescein angiography showing hyperfluorescence due to leakage of the retinal posterior pole lesion (middle left) and other temporal inferior hemangiomas partially treated with laser photocoagulation (middle right). Inferior: Infrared reflectance and Optical coherence tomography picture showing the intraretinal location of the lesion, confirming the diagnosis of an incipient retinal capillary hemangioma.
Treatment
For small retinal capillary hemangiomas (up to 500μm) with no exudation or subretinal fluid, careful observation is recommended, especially for juxtapapillary lesions that tend to remain stable and have higher risk of optic nerve damage by destructive laser treatments. For peripheral tumors, treatment may be considered, since the local side effects are reduced and since enlargement of the lesions may occur making them more difficult to treat with laser or cryotherapy.22,23
For hemangiomas up to 4.5mm, laser applied over several sessions is effective for 91% to 100% of the cases, but it is most effective in tumors that are 1.5 mm or smaller. Photocoagulation can be performed on the tumor or on the feeder artery, or on both.24 For juxtapapillary sessile or exophytic lesions, the laser burns should be intense enough to coagulate the entire retinal thickness. For this reason, treatment can be hazardous to the optic nerve and major retinal vessels.25 In these cases, photodynamic therapy may be the preferred alternative due to less risk of optic nerve damage.26
For hemangiomas larger than 4mm, there is poor response to laser and cryotherapy. In such cases, more aggressive approaches with plaque radiotherapy or external beam radiation should be tried.
Unfortunately, cases of large retinal capillary hemangiomas may progress to traction or rhegmatogenous retinal detachments and pars plana vitrectomy may be necessary.3
Genetic Counseling
Currently there is no method of gamete selection to prevent Von Hippel Lindau disease in patients’ siblings. The genetic studies injure the gametes.
Once the disease causing mutation in the family is known, prenatal testing may be performed on cells obtained by chorionic villus sampling that is performed in between 10-11 weeks' gestation, or as a preimplantation genetic diagnosis.27 Hence, the standard of care is genetic testing to detect gene mutations in siblings of patients with the disease. More recent techniques (next generation sequencing) to detect mosaicism (mutation restricted to only a few cells of the body) have improved detection of mutated alleles in patients with suspected Von Hippel disease.28
Early detection of gene carriers of the disease and subsequent regular exams including screening for kidney, pancreas, inner ear, retinal and cerebellum tumors are imperative for early detection of these lesions. This improves the chances of successful treatment and consequently better quality of life for VHL patients.29
Latest Management Developments
Intravitreal Anti-VEGF
Anecdotal reports have mentioned the use of anti-VEGF agents alone30 or in combination with photodynamic therapy for juxtapapillary retinal capillary hemangioma treatment, resulting in resolution of hard exudates and subretinal fluid and, in some cases, involution of the retinal tumor.31 The use of pegaptanib and bevacizumab has been studied in a small series of cases, where reduction in hard exudates and retina thickness was verified, with and without changes on hemangioma size, making results inconclusive.32
Oral Propanolol
Propranolol is a nonselective beta-2 blocker whose precise mechanism of action on retinal capillary hemangiomas is unclear. One of the main routes related to its effect is related to the inhibition of Hypoxia Inducible Factor (HIF). In fact, experiments performed with cells isolated from cerebral hemangioblastomas showed that transcription from genes activated from the hypoxia inducible factor was reduced about 40% compared to the controls after topical treatment with propranolol.33 These experiments suggest that by inhibiting HIF, there is reduced genesis of vascular endothelial growth factor, fibroblast growth factor and metalloproteases, and consequently angiogenesis.34 Despite the basic evidence, an anecdotal case report did not show significant change on retinal capillary hemangioma after propranolol treatment.35
References
- Goldberg RE, Pheasant TR, Shields JA. Cavernous hemangioma of the retina. A four-generation pedigree with neurocutaneous manifestations and an example of bilateral retinal involvement. Arch Ophthalmol. 1979;97(12): 2321-2324.
- Gass JD. Developmental Tumors of the Retinal Pigment Epithelium (RPE) and Retina. Stereoscopic Atlas of Macular Diseases, diagnosis and treatment. J. D. Gass. St. Louis, Missouri, Mosby. II: 1997:1061.
- Singh AD, Rundle PA, Rennie I. Retinal vascular tumors. Ophthalmol Clin North Am. 2005;18(1):167-176, x.
- Kitzmann AS, Pulido JS, Ferber MJ, Highsmith WE, Babovic-Vuksanovic D. A splice-site mutation in CCM1/KRIT1 is associated with retinal and cerebral cavernous hemangioma. Ophthalmic Genet. 2006;27(4):157-159.
- Gass JD. Cavernous hemangioma of the retina. A neuro-oculo-cutaneous syndrome. Am J Ophthalmol. 1971;71(4):799-814.
- Reddy S, Gorin MB, McCannel TA, Tsui I, Straatsma BR. Novel KRIT1/CCM1 mutation in a patient with retinal cavernous hemangioma and cerebral cavernous malformation. Graefes Arch Clin Exp Ophthalmol. 2010;248(9): 1359-1361.
- Messmer E, Font RL, Laqua H, Höpping W, Naumann GO. Cavernous hemangioma of the retina. Immunohistochemical and ultrastructural observations. Arch Ophthalmol. 1984;102(3): 413-418.
- Shields JA, Eagle RC Jr, Ewing MQ, Lally SE, Shields CL. Retinal cavernous hemangioma: fifty-two years of clinical follow-up with clinicopathologic correlation. Retina 2014;34(6): 1253-1257.
- Pastor-Idoate S, Gil-Martinez M, Crim N, et al. Swept-source optical coherence tomography of retinal cavernous hemangioma: a new imaging modality. J Pediatr Ophthalmol Strabismus. 2015;52 Online: e4-7.
- von Hippel E.Uber eine sehr self seltene Erkrankung der Netzhaut.Graefes Arch Ophthalmol. 1904;59:83–106.
- Lindau, A.Studien über kleinhirncysten: bau, pathogenese, und beziehungen zur angiomatosis retinae.Acta Pathol Microbiol Scandinavica. 1926;1:1–128.
- Melmon KL, Rosen SW. Lindau's Disease. Review of the Literature and Study of a Large Kindred. Am J Med. 1964;36: 595-617.
- Grossniklaus HE, Thomas JW, Vigneswaran N, Jarrett WH 3rd. Retinal hemangioblastoma. A histologic, immunohistochemical, and ultrastructural evaluation. Ophthalmology. 1992;99(1):140-145.
- Miyazawa A, Inoue M, Hirakata A, Okada AA, Iihara K, Fujioka Y. Expression of inhibin alpha by stromal cells of retinal angiomas excised from a patient with von Hippel-Lindau disease. Jpn J Ophthalmol. 2009;53(5): 501-505.
- Gass JD, Braunstein R. Sessile and exophytic capillary angiomas of the juxtapapillary retina and optic nerve head. Arch Ophthalmol. 1980;98(10):1790-1797.
- Stolle C, Glenn G, Zbar B, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12(6): 417-423.
- Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21(2): 163-167.
- Ceccaroli C, Pulliero A, Geretto M, Izzotti A. Molecular fingerprints of environmental carcinogens in human cancer. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33(2): 188-228.
- Baylin SB. DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol. 2005;2 Suppl 1: S4-11.
- Ponnaluri VK, Vavilala DT, Prakash S, Mukheri M. Hypoxia mediated expression of stem cell markers in VHL-associated hemangioblastomas. Biochem Biophys Res Commun. 2013;438(1): 71-77.
- Slim E, Antoun J, Kourie HR, Schakkal A, Cherfan G. Intravitreal bevacizumab for retinal capillary hemangioblastoma: A case series and literature review. Can J Ophthalmol. 2014;49(5): 450-457.
- Gass JD. Treatment of retinal vascular anomalies. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(3 Pt 1): OP432-442.
- Shields JA. Response of retinal capillary hemangioma to cryotherapy. Arch Ophthalmol. 1993:111(4): 551.
- McCabe CM, Flynn HW Jr, Shields CL, et al. Juxtapapillary capillary hemangiomas. Clinical features and visual acuity outcomes. Ophthalmology.2000;107(12): 2240-2248.
- Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109(10): 1799-1806.
- Wittenberg L. Ma P. Treatment of a von Hippel-Lindau retinal capillary hemangioma with photodynamic therapy. Can J Ophthalmol 2008;43(5): 605-606.
- Binderup M, Bisgaard ML, Harbud V, et al. Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Dan Med J 2013;60(12): B4763.
- Coppin L, Grutzmacher C, Crépin M, et al. VHL mosaicism can be detected by clinical next-generation sequencing and is not restricted to patients with a mild phenotype. Eur J Hum Genet. 22(9): 1149-1152.
- Rasmussen A, Alonso E, Ochoa A, et al. Uptake of genetic testing and long-term tumor surveillance in von Hippel-Lindau disease. BMC Med Genet. 2010;11: 4.
- Chelala E, Dirani A, Fadlallah A. Intravitreal anti-VEGF injection for the treatment of progressive juxtapapillary retinal capillary hemangioma: a case report and mini review of the literature. Clin Ophthalmol. 2013;7:2143-2146.
- Matušková V,. Vysloužilová. [The Use of anti-VEGF preparations and PDT in the treatment of retinal juxtapapillary hemangioma - a case report]. Cesk Slov Oftalmol. 2014:70(5): 196-200.
- Dahr SS, Cusick M, Rodriguez-Coleman H, et al. Intravitreal anti-vascular endothelial growth factor therapy with pegaptanib for advanced von Hippel-Lindau disease of the retina. Retina. 2007;27(2): 150-158.
- Albiñana V, Villar Gómez de Las Heras K, Serrano-Heras G, et al. (2015). Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet J Rare Dis. 10(1): 118.
- Flamme I, Krieg M, Plate KH. Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2alpha. Am J Pathol. 1998;153(1): 25-29.
- Tanabe H, Ishida M, Takeuchi S. [Two cases of retinal hemangioma treated by transpupillary thermotherapy]. Nihon Ganka Gakkai Zasshi. 2006;110(7): 525-531.