EPIDEMIOLOGY
Global Information
- Uveal melanoma is the most commonly diagnosed primary intraocular malignancy in adults
- 90% of uveal melanomas involve the choroid
- 5% arise in the ciliary body
- 5% develop in the iris
- Uveal melanomas account for 5-10% of all melanomas
- Childhood incidence is rare and increases with age, peaking at about 60 years
- There is no known gender predilection
- 30%-40% of all uveal melanomas are asymptomatic at the time of detection, being discovered as a result of routine ocular exam.
- Incidence are higher in more fairly pigmented groups but exceedingly lower in Asian and black populations.
DIFFERENTIAL DIAGNOSIS
- Choroidal nevus (Figure 1)
- Disciform scar (Figure 2)
- Congenital hypertrophy of the RPE (CHRPE) (Figure 3)
- Choroidal hemangioma (Figure 4)
- Melanocytoma (Figure 5)
- Choroidal osteoma (Figure 6)
- Metastatic carcinoma
- Hemorrhagic detachment of the choroid or retinal pigment epithelium (RPE)
PATHOPHYSIOLOGY/DEFINITION
Risk Factors
- Fair Complexion
- Ocular or oculodermal melanocytosis
- Genetic factors (most cases are sporadic however)
Pathology
- Classified according to modified Callender classification, with spindle cell, epithelioid cell, or mixed-cell (mixture of spindle and epithelioid cells) tumors
- Spindle-cell melanomas have the best prognosis
- Epithelioid melanomas have the worst prognosis
- Activating mutations in GNAQ and GNA11 are present in over 80% of primary uveal melanomas
SIGNS/SYMPTOMS
- Fundoscopic exam: elevated, dome-shaped subretinal mass (Figures 7,8), which may acquire a more mushroom shape if broken through the Bruch membrane
- Pigmentation can range from completely amelanotic to darkly pigmented
- May have associated serous retinal detachment
- May have orange pigmentation at the level of the RPE
Diagnostic Tools
- Slit-lamp biomicroscopy (SLB): SLB allows for assessment of anterior involvement of the tumor. Gonioscopy may be used at the slit lamp to aid in visualizing the tumor.
- Indirect ophthalmoscopy (IO): Gold standard for diagnosis. IO allows for 3D viewing of the choroidal lesion, as well as good views of the periphery.
- A-scan ultrasonography: medium to low internal reflectivity (Figure 9)
- B-scan ultrasonography: dome or mushroom-shaped choroidal mass. Anterior border is highly reflective, but mass is acoustically hollow, giving a dark appearance within the tumor (Figure 10)
- OCT reveals hyperreflective choroidal lesions and, in some cases, associated subretinal fluid
- Fluorescein angiogram (FA) may reveal double circulation (both retinal and choroidal circulation), especially in amelanotic melanomas and melanomas that have broken through the Bruch membrane
- CT or MRI if opaque media precludes clinical exam
- Positron emission tomography (PET)/CT scan has shown high sensitivity for finding liver metastasis at time of diagnosis
- The Collaborative Ocular Melanoma Study (COMS) reported 99% accuracy in the clinical diagnosis of choroidal melanoma
Classification
- Size (according to COMS):
- Small: 1.0-2.4 mm height and/or 4.0-8.0 mm diameter
- Medium: 2.5-10.0 mm height and/or 6 -16 mm diameter
- Large: >10.0 mm height and/or >16 mm diameter
- Cell type: Spindle, epithelioid, mixed
- Tumor, Node, Metastasis (TNM) system determined by the American Joint Committee on Cancer (AJCC) is used for tumor staging and prognosis
MANAGEMENT
Prognosis
- Tumor size is the best indicator of mortality.
- The COMS demonstrated a melanoma-specific mortality of 1% at 5 years in patients with small tumor size
- Cell type is also prognostic: epithelioid cell type is associated with a worse prognosis than spindle cell type
- Ciliary body involvement and extracellular extension are associated with worse prognosis
- Tumors with monosomy 3 are more likely to metastasize, and carry a worse prognosis, as do tumors with somatic BRCA1-associated protein (BAP1) inactivation. Gene profiling can classify tumors into class 1 tumors (low risk of metastasis) or class 2 tumors (high risk of metastasis)
- While local control may be achieved, micrometastatic disease at the time of diagnosis of the primary tumor results in a high rate of metastatic disease, any time from the time of diagnosis of the primary tumor to decades later. According to COMS, the 5-year and 10-year rates of metastatic disease was 25% and 34%, respectively
- Metastatic disease most commonly occurs first in the liver (95%)
- Other sites of metastasis include lungs, bones, and skin, though metastatic lesions at a variety of atypical sites have been documented
- Median survival in metastatic disease to the liver is between 4-6 months
Treatment
- Options include enucleation, as well as more conservative treatments, including: ionizing radiation, radioactive plaque brachytherapy, external-beam radiation, charged-particle therapy, stereotactic radiation therapy, and gamma-knife radiosurgery, tumor excision (higher risk of complications, and dissemination or recurrence of the tumor), photodynamic therapy, transpupillary thermotherapy (TTT), and scleral diathermy,
- The COMS is a multicenter study on choroidal melanoma from which valuable information regarding treatment and prognosis has been obtained
- COMS small tumor trial: observational study identifying risk factors for tumor growth: These included:
- Tumor thickness and basal diameter
- Presence of orange pigmentation
- Absence of drusen or RPE changes
- Presence of pinpoint hyperfluoresence on FA
- COMS medium-size tumor trial: enucleation and radioactive plaque brachytherapy found no difference in survival for up to 12 years after treatment
- COMS large-size tumor trial: external-beam radiation performed prior to enucleation confers no benefit in 5-year survival compared to enucleation alone
- Long-term follow-up is necessary to assess for recurrence or complications from prior therapy (eg, radiation retinopathy)
- Surveillance for metastatic disease should be considered. Although there is no universally accepted algorithm, screening often includes:
- liver ultrasound
- liver function tests
- chest x-ray
- Further testing, including: CT scan, MRI, and PET/CT scan as needed for any abnormalities
- Studies, however, demonstrate no significant improvement in mortality between asymptomatic diagnosis of metastatic disease from surveillance testing or symptomatic diagnosis, primarily due to the current lack of effective therapies for metastatic disease
- Locally recurrent melanoma is often treated with enucleation, or enucleation plus radiation therapy, depending on the extent of recurrence
- For metastatic disease, treatment is currently limited and mortality is high. Therapeutic options include:
- resection of metastatic nodules
- hepatic intra-arterial chemotherapy
- intra-arterial hepatic chemoembolization
- immunoembolization, systemic chemotherapy
- targeted therapy (i.e. monoclonal antibodies)
- immunotherapy
CASE STUDY
Age
Gender
History of present illness
- Patient presented for evaluation of blurry vision in the right eye for the last 3 months
Past Medical History
Family History
Physical Exam:
- Visual acuity was 20/800 OD, 20/20 OS
- Dilated fundus examination revealed an elevated mushroom-shaped pigmented lesion in the inferior macula, approximately 8.5mm in diameter, with associated subretinal fluid.
- B-scan ultrasonography revealed medium internal reflectivity and an anteroposterior height of 4 mm.
- A diagnosis of choroidal melanoma was made.
Treatment
- This was a medium-sized tumor (based on COMS) at the time of diagnosis.
- Options for treatment included radiation (preferred therapy; plaque radiation or external-beam radiation), transpupillary thermotherapy (TTT), local resection, or enucleation.
- Large tumors warrant enucleation.
- Small tumors may be treated with radiation, transpupillary thermotherapy, resection (if the above options are not available), and less commonly, with enucleation (or if the above options are not available).
- In this case, radiation therapy was not available locally and the patient was unable to travel; therefore, TTT versus local resection were considered.
- The patient underwent TTT with close follow-up thereafter.
- Two months after thermotherapy, tumor growth was noted; therefore, an enucleation was performed.
- No local recurrence was noted.
- The patient was followed closely with liver function tests annually thereafter.
- The cost of annual surveillance radiographic imaging (chest x-ray and liver ultrasound, which were available locally, or CT scan or MRI which were not available locally) was prohibitive and was therefore not pursued.
- Of note, previous studies indicate no survival benefit with diagnosis of metastases from surveillance imaging due to the current lack of effective therapy for metastatic uveal melanoma. However, in regions where enrollment in clinical trials for novel therapies (local resection or targeted molecular therapies) are available, imaging as discussed above should also be considered, as earlier diagnosis may allow resection of solitary metastastic lesions or enrollment in a clinical trial.
IMAGE LIBRARY
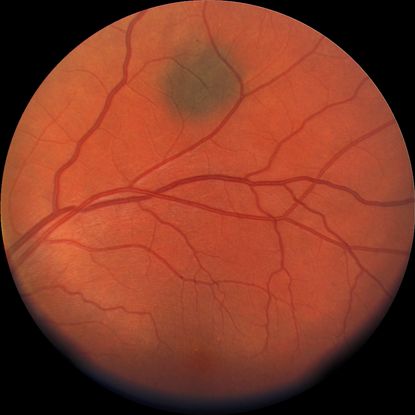
Figure 1. Flat choroidal nevus.
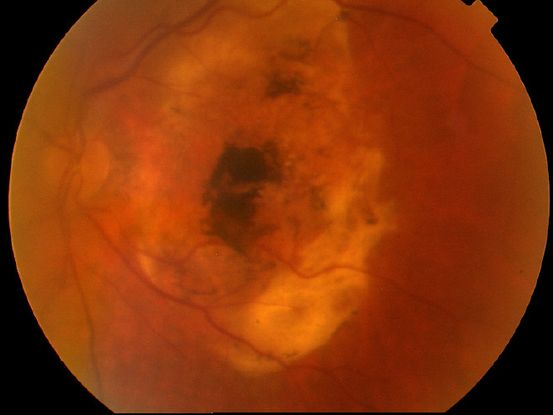
Figure 2. Disciform scar.
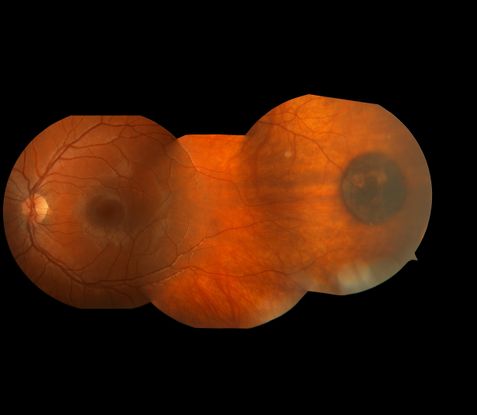
Figure 3. CHRPE lesion in an 11 year-old female.
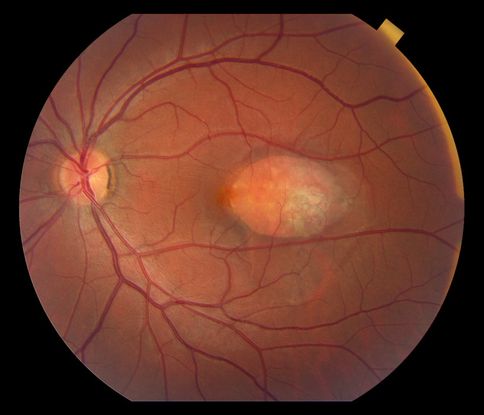
Figure 4. Choroidal hemangioma.
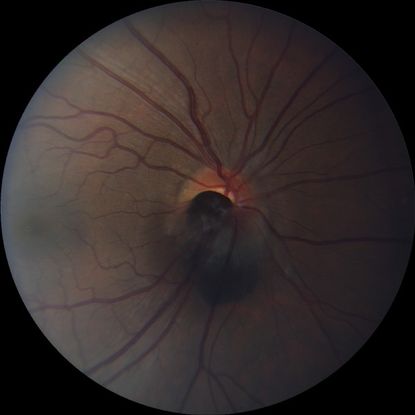
Figure 5. Optic disc melanocytoma.
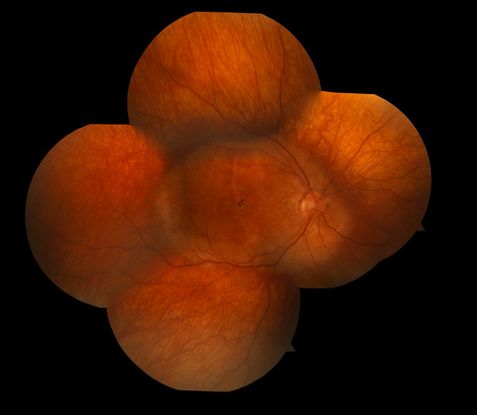
Figure 6. Choroidal osteoma.
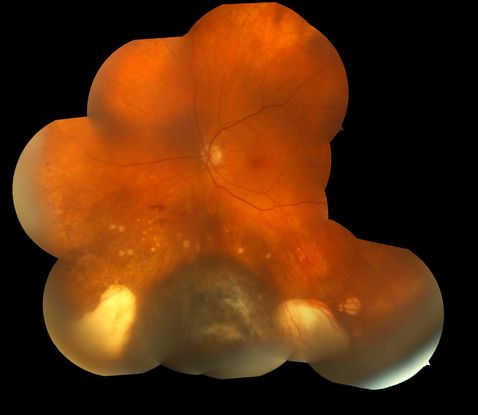
Figure 7. Darkly pigmented choroidal melanoma with surrounding areas of atrophic chorioretinal scarring.
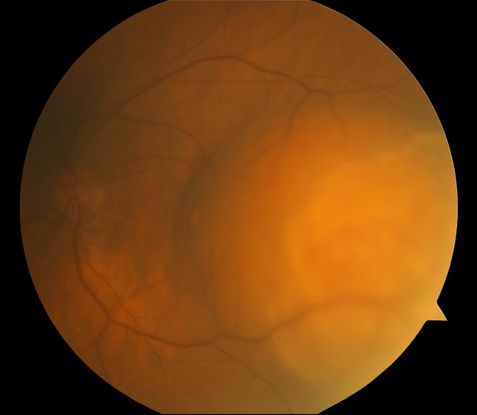
Figure 8. Amelanotic choroidal melanoma.
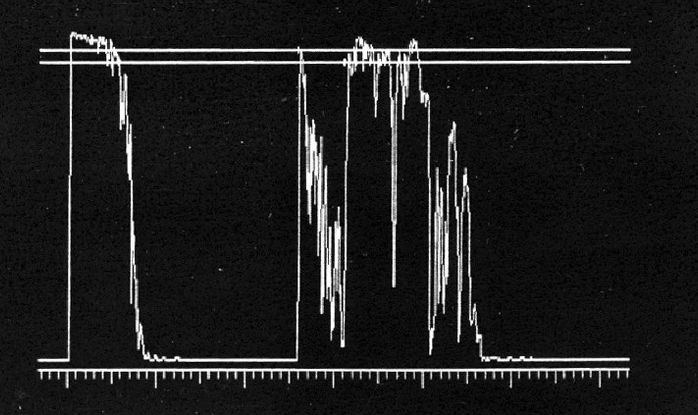
Figure 9. A-scan ultrasonography demonstrates high initial peak followed by low internal reflectivity.
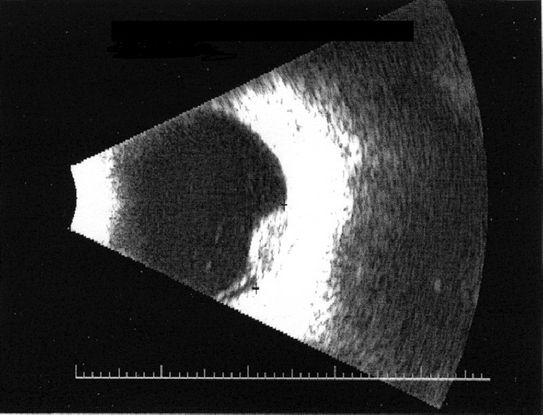
Figure 10. B-scan ultrasonography shows reflective anterior border with darker internal appearance.
REFERENCES
Basic and Clinical Science Course, Section 4. Ophthalmic Pathology and Intraocular Tumors. San Francisco: American Academy of Ophthalmology; 2013-2014: 274-288.
Cheng CY, Hsu WM. Incidence of eye cancer in Taiwan: an 18-year review. Eye. 2004; 18: 152-158.
Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma, II: initial mortality findings. COMS report No. 10. Am J Ophthalmol. 1998; 125: 779-796.
Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119(7): 969-982.
Diener-West M, Reynoles SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005; 123(12): 1639-1643.
Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 2005;140: 612-617.
Kaneko A. Japanese contributions to ocular oncology. Intl J Clin Oncol. 1999; 4: 321-326.
Kim IK, Lane AM, Gragoudas ES. Survival in patients with presymptomatic diagnosis of metastatic uveal melanoma. Arch Ophthalmol. 2010;128(7):871-5.
Kivelä T, Kujala E. Prognostication in eye cancer: the latest tumor, node, metastasis classification and beyond. Eye. 2013;27(2):243–52.
Landreville S, Agapova OA, Harbour JW. Emerging insights into the molecular pathogenesis of uveal melanoma. Future Oncol. 2008; 4(5): 629-636.
Margo CE. The Collaborative Ocular Melanoma Study: an overview. Cancer Contr. 2004; 11(5): 304-309.
Miller B, Abrahams C, Cole GC, et al. Ocular malignant melanoma in South African blacks. Br J Ophthalmol. 1981; 65: 720-722.
Pereira PR, Odashiro AN, Lim L-A, Miyamoto C, Blanco PL, Odashiro M, et al. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. 2013; 7: 1669–1682.
Shields JA. Current approaches to the diagnosis and management of choroidal melanoma. Surv Ophthalmol. 1977; 21: 443-463.
Tschentscher F, Prescher G, Zeschnigk M, Horsthemke B, Lohmann DR. Identification of chromosomes 3, 6, and 8 aberrations in uveal melanoma by microsatellite analysis in comparison to comparative genomic hybridization. Cancer Genet and Cytogenet. 2000; 122(1):13–17.
Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, Lutz JM, Paci E; EUROCARE Working Group. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114: 2309-2315.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editor:
Sub-Saharan Africa:
Dupe Ademola-Popoola, MBBS, FMCOphth, FWACS, Department of Ophthalmology, University of Ilorin
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2014 American Academy of Ophthalmology®. All Rights Reserved.