EPIDEMIOLOGY
Global Information
- Choroidal metastases are the most common intraocular tumors.
- 66% of patients have a known history of carcinoma at the time of diagnosis of the choroidal metastasis, while 34% present with no known history of cancer. In up to 10% of patients, no primary cancer is discovered even after workup.
- The most common primary tumor locations are the breast and lung (Table 1).
- Upon diagnosis of choroidal metastases, more than 50% of patients are found to have other sites of metastatic spread, most commonly the lung.
- Prognosis is poor with a median survival time of 6-12 months.
Table 1: Frequency of Primary Cancer Site in Men and Women with Metastasis to the Choroid
|
Women
|
Men
|
|
Breast (68%)
|
Lung (40%)
|
|
Lung (12%)
|
Unknown (29%)
|
|
Unknown (12%)
|
GI Tract (9%)
|
|
GI Tract (2%)
|
Prostate (6%)
|
|
Skin (1%)
|
Renal (6%)
|
|
Other (4%)
|
Breast (1%)
|
|
|
Other (4%)
|
Regional Information
Little region-specific data is available on the incidence of choroidal metastases geographically. The available data suggests that the primary cancer sites of choroidal metastases closely resemble the frequency of cancers in the population studied, ie, the most common cancers are also the most commonly associated with choroidal metastases. Thus, it is likely that the site of the primary tumor from choroidal metastases will vary geographically based on the cancers most frequently encountered in a given region.
DIFFERENTIAL DIAGNOSIS
PATHOPHYSIOLOGY/DEFINITION
- The mechanism of intraocular metastasis is via hematogenous dissemination of tumor cells.
- Arterial blood supply to the eye dictates the primary sites of tumor deposition within the eye.
- Given the rich vascular supply to the posterior choroid, it is the most favored site of intraocular metastasis.
- The posterior ciliary arteries provide a greater flow for tumor emboli to the posterior choroid than the fewer, lower flow anterior ciliary vessels to the anterior uvea (iris and ciliary body).
- Likewise, the optic nerve and retina are rarely exclusively involved, given they are supplied typically by a single vessel (central retinal artery).
SIGNS/SYMPTOMS
Medical history, clinical exam, and noninvasive testing are typically sufficient to reach a diagnosis.
Symptoms
The metastases are usually asymptomatic. However, depending on the location within the choroid, the patient may experience visual symptoms, most commonly blurred vision.
Fundoscopic Findings
- Elevated plaque-like choroidal lesions with indistinct borders
- Usually yellow in color
- Pink or bright orange if primary tumor is renal cell carcinoma, thyroid carcinoma
or carcinoid
- Brown or tan if metastatic melanoma
- Usually larger than the optic disc in diameter
- Over 90% of lesions are in the posterior pole
- Up to 50% of cases exhibit bilateral choroidal lesions, and almost 33% present with
more than one lesion in an eye
- Frequently associated with exudative retinal detachment (occurs in 88% of patients) (Figure 7)
Ultrasonography
- B-scan generally shows a subretinal echogenic mass with ill-defined borders
- A-scan generally shows mass with medium to high internal reflectivity, little vascularity, and a solid consistency
Fluorescein angiography (FA)
- Little intratumoral vascularity
- Blocked choroidal fluorescence in early phase, diffuse leaks in late phase
Other Testing Methods
- MRI and CT imaging have little usefulness in diagnosing a choroidal tumor. They may show a mass, but are not sufficiently sensitive or specific to be more helpful than a clinical examination.
- Fine-needle aspiration biopsy is contraindicated in intraocular neoplastic disease. It should be considered only if the primary tumor cannot be located (to obtain a diagnosis to facilitate appropriate treatment selection) and even then should be approached with caution.
MANAGEMENT
The treatment of choroidal metastasi s requires a multidisciplinary team composed of an ophthalmologist, oncologist, and radiation oncologist.
The diagnosis of choroidal metastasi s of a known cancer should prompt an appropriate work up to restage the disease and treat other sites of metastases appropriately. In the majority of cases of choroidal metastases, other sites of metastases are also found, most commonly the lung.
- In as many as 34% of cases of choroidal metastasi s, there is no known primary cancer at the time of diagnosis.
- Initiation of appropriate systemic chemotherapy or other targeted therapies to treat the primary tumor is frequently the first step in management of choroidal metastases. Because the choroid is external to the blood-aqueous and blood-retinal barriers, penetration of systemic agents to the choroid is usually good, especially due to the highly fenestrated nature of the choroidal vasculature. Moreover, such systemic therapies concurrently treat other sites of metastases, which are frequently noted at the time of diagnosis of the choroidal metastasis.
- Local therapies may be more appropriate if the choroid is the only site of metastases, or if the choroidal lesions fail to respond to appropriate systemic chemotherapies.
TREATMENT
The following local treatment modalities are used depending on availability:
- Radiation therapy (by far the treatment of choice)
- External-beam radiation
- Proton beam radiation
- Brachytherapy (Figure 8)
- Transpupillary thermotherapy (TTT)
- Laser photocoagulation (Figure 7)
- Photodynamic therapy
- Anti-VEGF agents such as bevacizumab (very limited early data from case studies and
series)
- Local resection
- Enucleation (in cases of severe, unrelenting pain)
The extent of metastatic disease is classified as diffuse, focal, and local. The treatment options depend on this classification:
- Diffuse metastatic disease
- Chemotherapy
- Hormonal therapy (for hormone-dependent tumors)
- Immune modulation
- Anti-VEGF agents
- Hospice care for terminally ill
- Focal disease
- Local disease
- Surgical treatment for painful eyes with secondary glaucoma
IMAGE LIBRARY
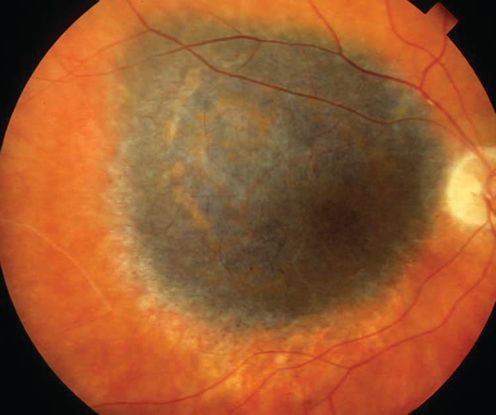
Figure 1. Choroidal melanoma. (© 2013 American Academy of Ophthalmology)From One Network Images, http://one.aao.org/image/choroidal-melanoma-4
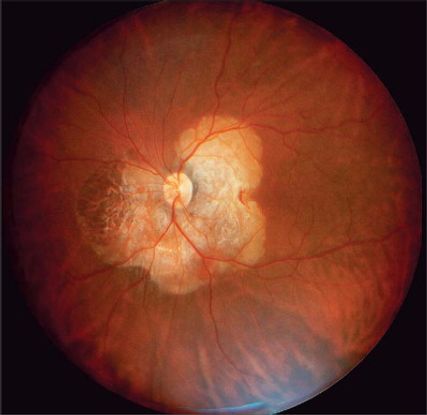
Figure 2. Choroidal osteoma. (Reprinted, with permission, from Albert DM, Miller JW, Azar DT, Blodi BA, Cohan JE, Perkins T. Albert & Jakobiec's Principles & Practice of Ophthalmology. 3rd ed. Philadelphia: WB Saunders; 2008.)
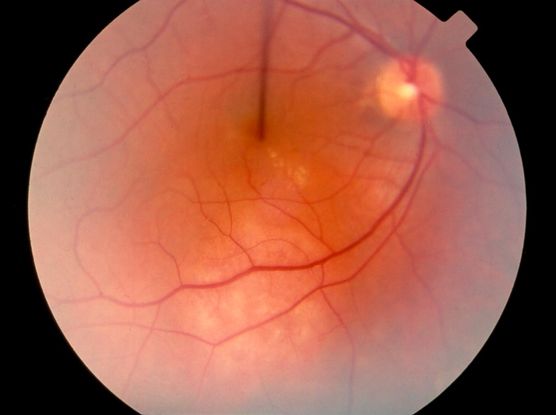
Figure 3. Choroidal hemangioma. (© 2013 American Academy of Ophthalmology) From One Network Images, http://one.aao.org/image/choroidal-hemangioma-2
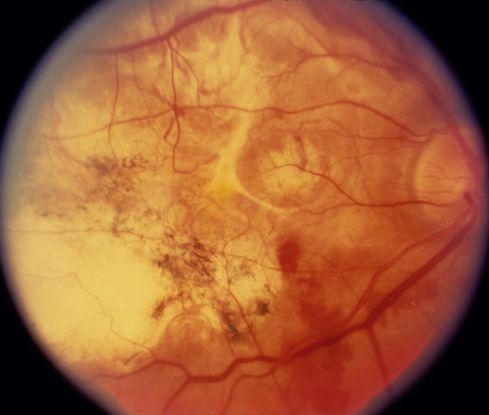
Figure 4. Disciform scar. (© 2013 American Academy of Ophthalmology) From One Network Images, http://one.aao.org/image/disciform-scarring-2
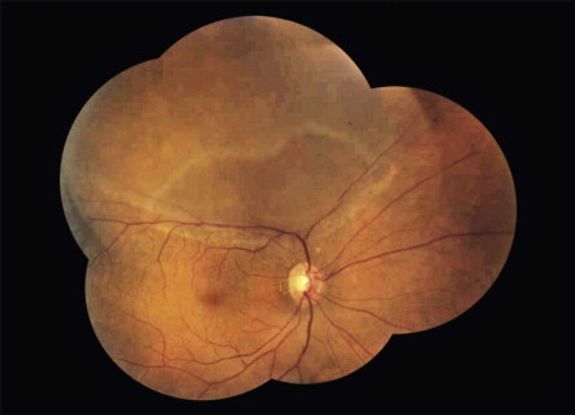
Figure 5. Rhegmatogenous retinal detachment. From Rao et al. Internal limiting membrane peeling for primary rhegmatogenous retinal detachment repair. Ophthalmology. 2013; 120(5): 1102-1103.
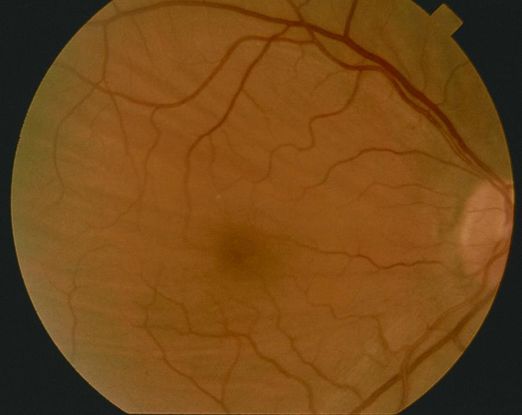
Figure 6. Posterior scleritis (© 2013 American Academy of Ophthalmology) From One Network Images, http://one.aao.org/image/scleritis-13
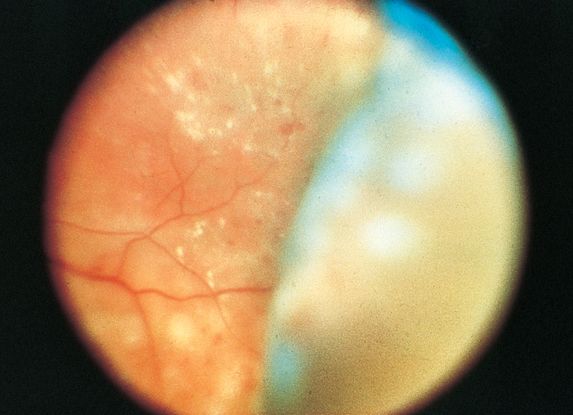
Figure 7. Choroidal detachment following panretinal photocoagulation for the management of diabetic retinopathy. (Image courtesy of M. Gilbert Grand, MD.) From BCSC Sec 12/ONE image library.
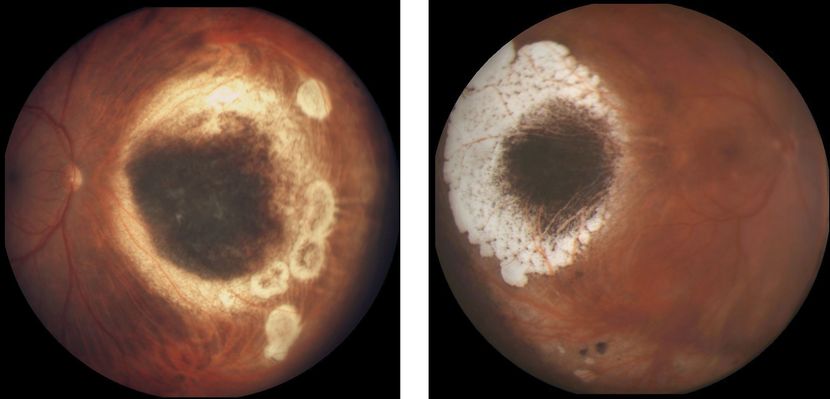
Figure 8. Choroidal melanoma treated by radioactive brachytherapy. (Left) Mildly elevated remnants of melanoma surrounded by atrophic chorioretinal scarring, nasal to the optic nerve head. (Right) Flat remnants of melanoma pigmentation surrounded by chorioretinal scarring located temporal to the macula. (Images courtesy of Jacob Pe’er, MD.) From BCSC/ONE image library.
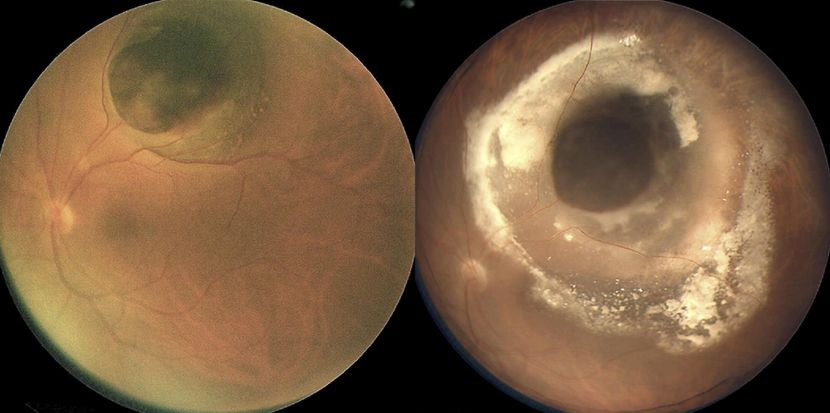
Figure 9. (Left) Mushroom-shaped pigmented choroidal melanoma with initial thickness of 7.5 mm before iodine-125 plaque radiotherapy. (Right)There is surrounding lipid exudation and serous RD 16 months after plaque radiotherapy; note involvement of the fovea by lipid exudation accounting for visual acuity of 20/200. (Reproduced from Mashayekhi A, Tuncer S, Shields CL, Shields JA. Tumor-related lipid exudation after plaque radiotherapy of choroidal melanoma: the role of Bruch’s membrane rupture. Ophthalmology. 2010; 117 (5): 1013-1023.)
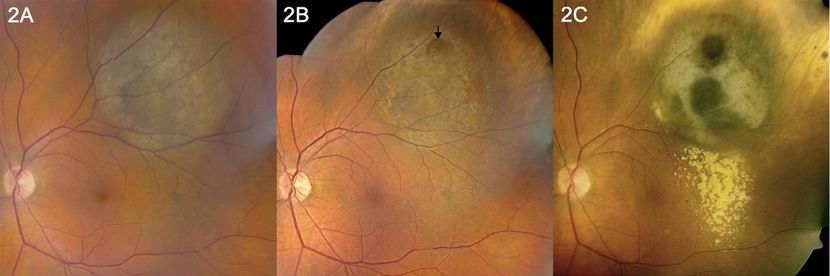
Figure 10. (Left) Pigmented choroidal melanoma with initial thickness of 5.0 mm and no visible Bruch's membrane rupture at initial presentation; FNAB of tumor for cytogenetic studies was performed at time of application of iodine-125 radioactive plaque. (Middle) Four months after plaque radiotherapy, focal area of Bruch's membrane rupture is visible at site of needle biopsy. (Right) Nine months after radiotherapy, lipid exudation is noticeable at the inferior margin of the tumor; there is radiation-induced chorioretinal atrophy at the anterior and lateral margins of the tumor. FNAB = fine-needle aspiration biopsy. (Reproduced from Mashayekhi A, Tuncer S, Shields CL, Shields JA. Tumor-related lipid exudation after plaque radiotherapy of choroidal melanoma: the role of Bruch’s membrane rupture. Ophthalmology. 2010; 117 (5): 1013-1023.)
REFERENCES
Albert DM, Rubenstein RA, Scheie HG. Tumor metastasis to the eye. I. Incidence in 213 adult patients with generalized malignancy. Am J Ophthalmol. 1967; 63: 723-726.
Albert D, Miller J, Azar D, Blodi B, Cohan J, Perkins T. Albert & Jakobiec's Principles & Practice of Ophthalmology. 3rd ed. Philadelphia: WB Saunders; 2008: 4943-4948.
Chen CJ, McCoy AN, Brahmer J, et al. Emerging treatments for choroidal metastases. Surv of Ophthalmol. 2011; 56(6): 511-521.
Giuliara GP, Sadaka A. Uveal metastatic disease: current and new treatment options. Oncol Rep. 2012; 27(3): 603-607.
Jiang K, Brownstein S, Sekhon HS, et al. Ocular metastasis of lung adenocarcinoma with ELM4-ALK translocation: A case report with a review of the literature. Saudi J. Ophthalmol. 2013; 27(3): 187-192.
Kanski J, Bowling B. Synopsis of Clinical Ophthalmology. 3rd ed. Philadelphia: Elsevier Ltd; 2013; 217-239.
Ryan S, Sadda S, Hinton D, Schachat A, Wilkinson C, Wiedemann P. Retina. 5th ed. Philadelphia: Elsevier ; 2013: 2324-2329.
Shields CL, Shields JA, Gross NE, et al. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104: 1265‑1276.
Shields JA, Shields CL, Singh AD. Metastatic neoplasms in the optic disc: the 1999 Bjerrum Lecture: part 2. Arch Ophthalmol. 2000; 118: 217-224.
Singh A, Damato B, Pe’er J, Murphree A, Perry J. Clinical Ophthalmic Oncology.
Philadelphia: Elsevier Publishing Company; 2007: 322-327.
Weiss L et al. Analysis of the incidence of intraocular metastasis. Br J Ophthalmol. 1993; 77: 149-151.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editor:
Latin America:
Sandra Belalcázar Rey, MD, Universidad del Rosario, Bogota, Colombia
Lihteh Wu, MD, Department of Ophthalmology, Vitreoretinal Section, Instituto De Cirugia Ocular, San José, Costa Rica
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2014 American Academy of Ophthalmology®. All Rights Reserved.