EPIDEMIOLOGY
Global Information
Fuchs endothelial corneal dystrophy (FECD) is a common indication for corneal transplantation, accounting for approximately 20% of all penetrating keratoplasties.
Central guttae without corneal edema have been observed in 3.9%–9.6% of eyes in patients greater than age 40, and in approximately 10.5% of eyes in patients greater than age 60 (Eghrari et al, 2010).
Both early and late onset forms have female predominance at a ratio of 2.5:1 to 3:1.
Regional Information (LATIN AMERICA)
- A retrospective study in Sao Paulo, Brazil, for the Cornea Transplantation Project reviewed the main indications of corneal transplantation. With 171 patients studied, Fuchs dystrophy was considered one of the lowest indications (1.9%) (Calix et al, 2006).
DIFFERENTIAL DIAGNOSIS
- Aphakic or pseudophakic bullous keratopathy
- If history of cataract surgery
- Congenital hereditary endothelial dystrophy
- Congenital hereditary stromal dystrophy
- Posterior polymorphous dystrophy
- Iridocorneal endothelial (ICE) syndrome (Figure 1)
- Hassal-Henle bodies
- Secondary causes of endothelial dysfunction (anterior uveitis, herpes simplex keratitis, interstitial keratitis)
PATHOPHYSIOLOGY/DEFINITION
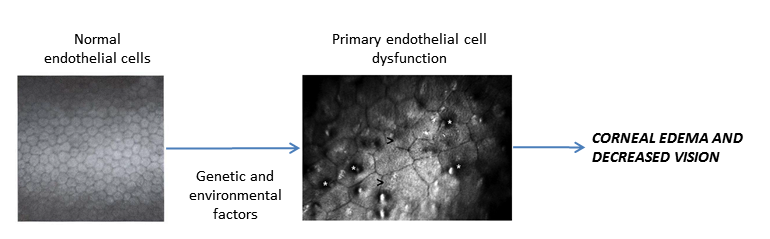
Chart 1. Pathophysiology. See Image Library for figures.
Definition
- Fuchs dystrophy is characterized as a late onset (age >50), slowly progressive disease with (1) decreased visual acuity in the morning that initially improves throughout the day, (2) diffuse corneal opacification, and (3) stromal edema.
- The disease is generally bilateral, but it may be asymmetric with one eye having more severe disease than the other.
- Figure 2 shows normal endothelial cells compared with guttae causing endothelial cell loss and change in Fuchs dystrophy.
Genetic Analysis
- Cases without known inheritance are most common.
- Genetic basis is complex and heterogeneous.
- Early-onset FECD has been mapped to COL8A2 (collagen VIII alpha-2).
- Investigations into the pathogenesis of FECD have suggested there may be a role for apoptosis in the endothelial cells in the progression of disease, and proteomic analyses implicate clusterin and transforming growth factor B-induced protein (TGFBIP) in the formation of guttae (Elhalis et al, 2010).
Suspected Mechanism
- Primarily based on dysfunction of endothelial cells, which manifests as increased corneal edema.
- Endothelial cells are responsible for maintaining the delicate hydration balance.
- Fluid accumulates in the corneal stroma and excess fluid migrates to the epithelium and collects in bullae.
- When the bullae rupture, irritation of the cornea can cause scar tissue and blood vessel formation.
- The stressed endothelial cells deposit collagen and extracellular matrix in Descemet membrane, which appear as dark bodies (guttae) in a thickened Descemet membrane. Guttae become buried and confluent or may be absent.
- Microscopically there is a reduced number of endothelial cells with various sizes (polymegathism) and more polymorphic (pleomorphism) with decreased hexagonality (Figure 3).
Stages of Fuchs Endothelial Dystrophy
| Table 2. Stages of Fuchs Endothelial Dystrophy |
| Stage |
Description |
| 1 |
Central, nonconfluent corneal guttae
Typically asymptomatic |
| 2 |
Corneal guttae coalesce
Endothelial cell thinning and enlargement
Loss of hexagonal shape
Painless decrease in vision and glare |
| 3 |
Stromal edema and/or bullae
Ruptured bullae: painful and can lead to scarring and infection |
| 4 |
Cornea: densely opaque and vascularized
Subepithelial fibrosis |
Associated Conditions:
- Cataract
- Keratoconus
- Age-related macular degeneration
- Cardiovascular disease
SIGNS/SYMPTOMS
Signs
- Corneal guttae on Descemet membrane (develop centrally first then spread peripherally) (Figure 4a)
- Fine pigment dusting on endothelium (Figure 4b)
- Endothelial decompensation (beaten metal–like appearance) and corneal stromal edema (Figure 5)
- Microcystic changes in the epithelium (best seen after fluorescein) (Figure 6)
- Central epithelial edema and bullae (Figure 7 and Figure 8)
- Descemet folds and increased thickness (Figure 9 and Figure 10)
- Bullous keratopathy
- Subepithelial fibrosis and scarring (later stages)
- Peripheral superficial vascularization from chronic edema
Symptoms
- Photophobia
- Intermittent reduced vision, worse upon awakening
- Severe pain due to recurrent erosions from ruptured bullae
- Foreign body sensation
- Epiphora
- Progressive vision loss
MANAGEMENT
Evaluation
- Inquire about previous cataract surgery.
- Perform a slit-lamp examination.
- Guttae are best seen with retroillumination of specular reflection.
- Check intraocular pressure.
- Consider confocal microscopy to determine endothelial cell count
- Consider pachymetry to determine central corneal thickness
- Endothelial cell count <1000/mm2 or central corneal thickness > 640 μm suggests that cornea may decompensate with intraocular surgery.
Medical Therapy
- Prescribe topical sodium chloride 5% drops 4 times a day, ointment every night at bedtime.
- Apply warm air (eg, with a hairdryer) to the eye gently in morning to dehydrate the cornea.
- Reduce intraocular pressure with topical medications if pressure >20–22 mmHg
- Goal of lowering IOP is to reduce corneal edema.
- Treat ruptured corneal bullae as recurrent corneal erosion.
- Consider cycloplegic, antibiotic ointment, patching.
- If persistent or large epithelial defect, consider bandage contact lens
Surgery
- If there is significant visual impairment due to corneal scarring or edema, corneal grafting can be performed.
- Penetrating keratoplasty (PK) in Fuchs dystrophy has dropped from 13.6% in 2005 to 3.1% in 2014. PK requires a long period for visual rehabilitation, confers a high degree of postoperative astigmatism, and presents a lifelong risk of wound rupture.
- Since its introduction in 1998 endothelial keratoplasty procedures, such as Descemet stripping endothelial keratoplasty (DSEK) or Descemet stripping automated endothelial keratoplasty (DSAEK) and more recently Descemet membrane endothelial keratoplasty (DMEK), have become increasingly popular and are now the preferred treatment for isolated endothelial disease.
- In the United States in 2014, endothelial keratoplasty was the procedure of choice in more than 90% of the cases for the treatment of Fuchs endothelial dystrophy.
- A study comparing the 2 procedures demonstrated that DSEK is associated with fewer episodes of graft rejection (16% with PK compared to 5% with DSEK) and fewer episodes of graft failure within a 5-year follow-up period (Hjortdal et al, 2013).
- Beydoun et al (2015) analyzed the endothelial survival rate in 314 patients at 8 years finding better survival rates in eyes with FECD than in those with BK (survival probability, 0.97 [95%CI, 0.95-0.99] versus 0.84 [95%CI, 0.70-0.99], respectively) suggesting an excellent prognosis.
Note: The disease progresses slowly, and visual acuity remains good until epithelial or stromal edema develops.
CASE STUDY
History of Present Illness
A 66-year-old patient presents with fluctuating reduced vision in both eyes that has slowly progressed over the past 4 months. She notes a misty type of vision early in the morning that improves throughout the day. She endorses halos around sources of light. She denies any other past medical, surgical, or ocular history. Notably, her older sisters (age 75 and age 70) had a history of similar vision changes.
Examination
- Visual acuity: 20/30 in both eyes at distance and near
- Extraocular movements: Full
- Intraocular pressure: 17 mmHg in both eyes
- Pupils: 5 mm in dark, 2 mm in light, no relative afferent pupillary defect
- Visual fields: Full, both eyes
- Dilated fundus examination: normal macula, vessels, and periphery, both eyes
- Slit-lamp examination: confluent guttae, mild cataract, both eyes
A central corneal thickness measurement of 680 µm is obtained as well as a confocal endothelial cell count, which reveals 1200/mm2 with increased polymegathism and pleomorphism.
Treatment
- Given only mild corneal edema, the patient is started on topical sodium chloride 5% drops 4 times per day.
- The patient is informed that due to the endothelial cell dysfunction, when cataract surgery is considered she may first need a corneal graft or a combined corneal graft and cataract surgery procedure.
IMAGE LIBRARY
Differential Diagnosis
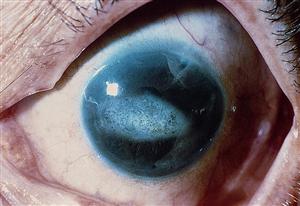
Figure 1. Classic iris changes seen in iridocorneal endothelial syndrome. (© 2015 American Academy of Ophthalmology, www.aao.org).
Pathophysiology/Definition
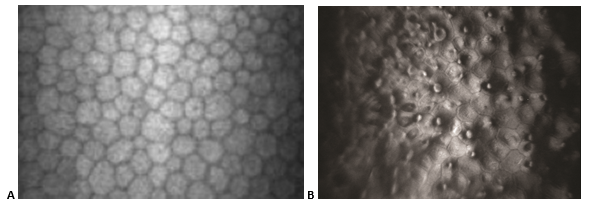
Figure 2. Fuchs dystrophy. A. In-vivo slit-lamp scanning confocal microscopy showing normal endothelial cells. B. Guttae causing endothelial cell loss and change in Fuchs dystrophy.). (Part A reproduced from Zhang J, Patel DV. The pathophysiology of Fuchs' endothelial dystrophy – a review of molecular and cellular insights. Exp Eye Res. 2015;130:97–105. with permission from Elsevier. Part B © 2015 American Academy of Ophthalmology, www.aao.org.)
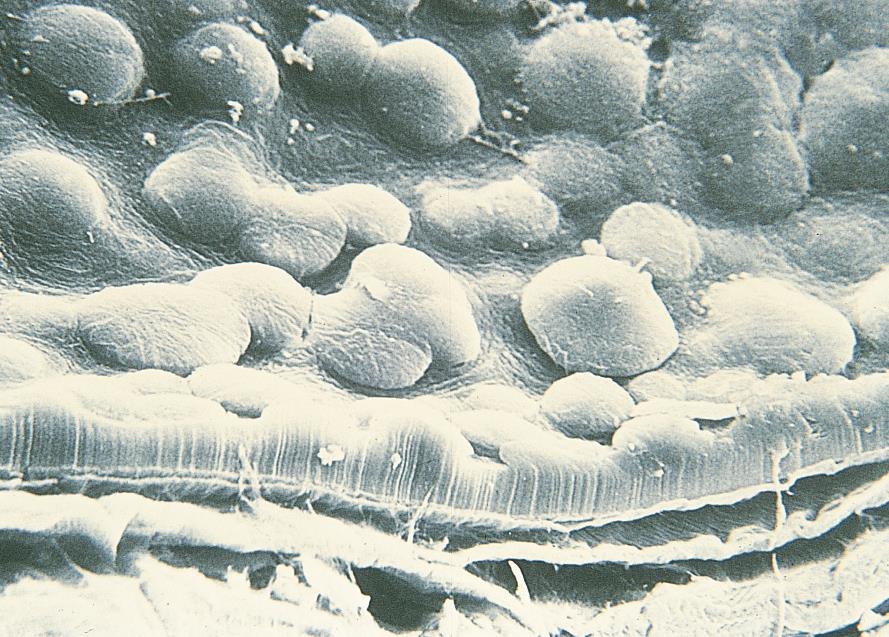
Figure 3. Scanning electron microscope image showing excrescences in corneal endothelium. (© 2015 American Academy of Ophthalmology, www.aao.org.)
Signs/Symptoms
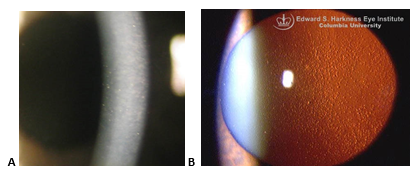
Figure 4. Fuchs endothelial dystrophy. A. Slit beam through cornea. Notice the imperfections (guttae) of the corneal endothelium on the left side of the beam. B. Multiple central corneal guttata (excrescences of Descemet membrane) associated with pigment dusting on the endothelium. (Part A reproduced, with permission, from Doan A, Lee A. Fuchs endothelial dystrophy: 35-year-old female with blurry vision OU lasting hours in the morning. EyeRounds Online Atlas of Ophthalmology. Part B reproduced, with permission, from Digital Reference of Ophthalmology. “Cornea & External Diseases.” Fuchs dystrophy.)
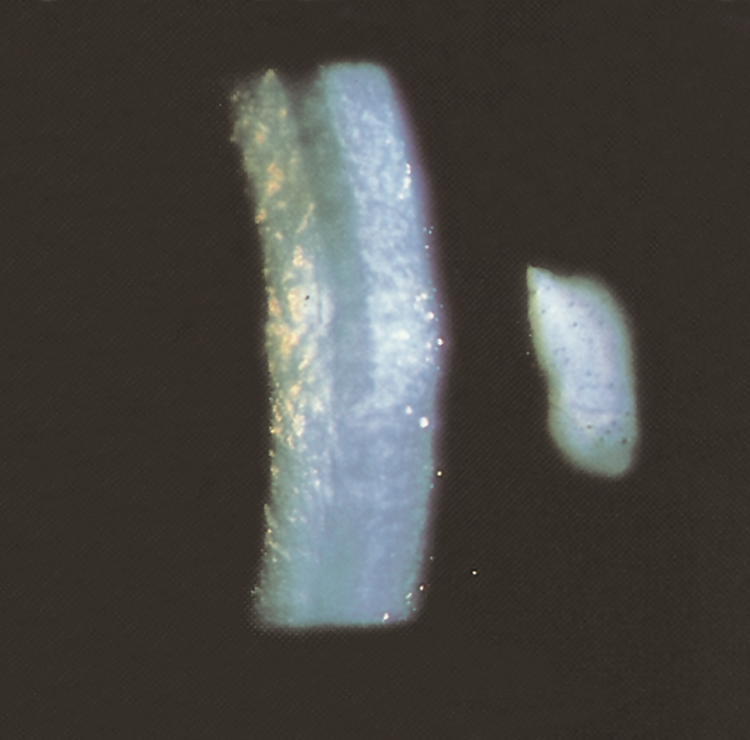
Figure 5. Advanced Fuchs endothelial dystrophy. Stromal edema, Descemet folds, and endothelial guttae. (© 2015 American Academy of Ophthalmology, www.aao.org).
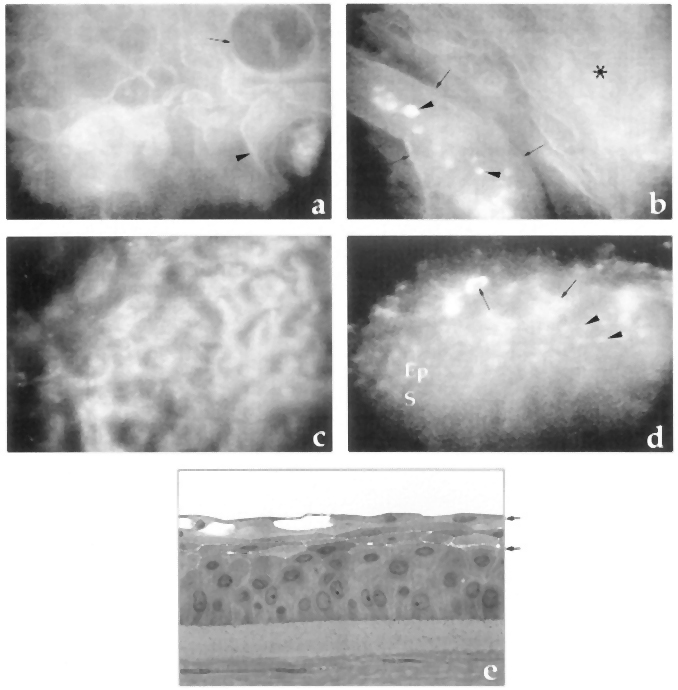
Figure 6. Microcystic changes in the epithelium. A. Cystic lesions in superficial epithelial cells with linear reflection (arrow) and irregular reflective image within the lesion (arrowhead). B. Highly reflective material (arrowheads) within an elongated bulla (arrows) and an abnormal epithelium as background (asterisk). C. Optical section at the endothelium showing guttae and enlarged endothelial cells. D. Oblique aspect of the epithelium in which normal stroma (S), epithelial cells (Ep, arrowheads), and irregular, edematous superficial cells (arrows) are observed. E. Semifine micrograph of the cornea of another patient with Fuchs dystrophy to correlate with previous images. (Reproduced, with permission, from Hernández-Quintela E, Mayer F, Dighiero P, et al. Confocal microscopy of cystic disorders of the corneal epithelium. Ophthalmology. 1998;105(4):631–6.)
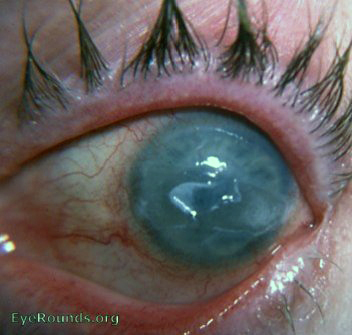
Figure 7. Fuchs epithelial endothelial corneal dystrophy with central ruptured epithelial bullae.
(Reproduced, with permission, from Caccamise WC. Fuchs epithelial-endothelial corneal dystrophy with central ruptured epithelial bullae. EyeRounds Online Atlas of Ophthalmology. )
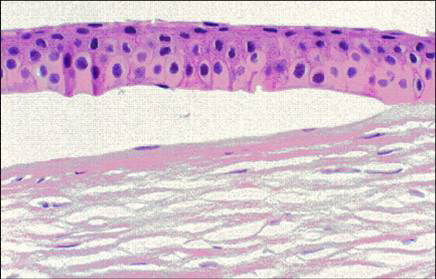
Figure 8. Fuchs corneal dystrophy. A subepithelial bulla resulting from a separation of the corneal epithelium from Bowman layer. (Reproduced from Klintworth GK. Corneal dystrophies. Orphanet Journal of Rare Diseases. 2009;4:7.)
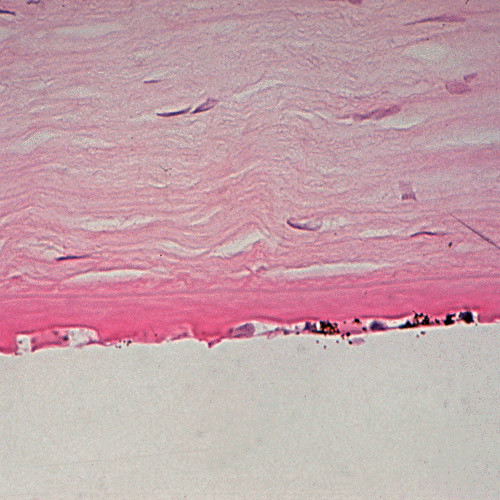
Figure 9. Fuchs corneal dystrophy. Light microscopic appearance of the corneal endothelium, Descemet membrane, and the adjacent corneal stroma. Hematoxylin and eosin stain. (Reproduced from Klintworth GK. Corneal dystrophies. Orphanet Journal of Rare Diseases. 2009;4:7.)
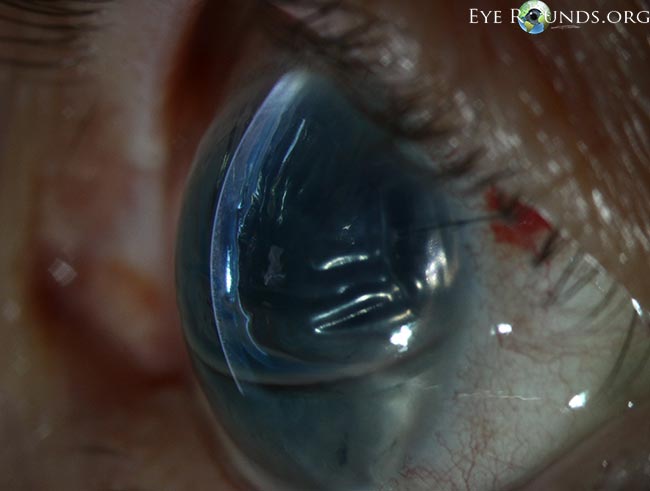
Figure 10. Corneal edema with Descemet membrane folds after DMEK. This patient had residual corneal edema with prominent Descemet membrane folds visible on the posterior corneal surface on her first postoperative day after undergoing Descemet's membrane endothelial keratoplasty (DMEK). A superotemporal limbal suture can be seen. After several days, the corneal edema eventually cleared and guttae were visible centrally in the graft tissue, consistent with Fuchs' endothelial dystrophy of the donor. (Reproduced, with permission, from Vislisel J. Corneal edema with Descemet’s membrane folds after DMEK. Image by Toni Venckus, CRA. EyeRounds Online Atlas of Ophthalmology. )
REFERENCES
Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38(2):149–68.
Al-Yousuf N, Mavrikakis I, Mavrikakis E, Daya SM. Penetrating keratoplasty: indications over a 10 year period. Brit J Ophthalmol. 2004;88(8):998–1001.
Baydoun L, Ham L, Borderie V, et al. Endothelial survival After Descemet membrane endothelial keratoplasty: effect of surgical indication and graft adherence status. JAMA Ophthalmol. 2015 Sep 10. doi: 10.1001/jamaophthalmol.2015.3064. [Epub ahead of print]
Borboli S, Colby K. Mechanisms of disease: Fuchs’ endothelial dystrophy. Ophthalmol Clin North Am. 2002;15(1):17–25.
Calix Netto MJ, Giustina ED, Ramos GZ, Peccini RF, Sobrinho M, de Souza LB. Major indications for corneal penetrating keratoplasty at a reference service in Sao Paulo state (Sorocaba – SP, Brazil). Arq Bras Oftalmol. 2006;69:661–4.
Cosar CB, Sridhar MS, Cohen EJ. Indications for penetrating keratoplasty and associated procedures, 1996–2000. Cornea. 2002;21(2):148–51.
Eghrari AO, Gottsch JD. Fuchs’ corneal dystrophy. Expert Rev Ophthalmol. 2010;5(2):147–59.
Elhalis H, Behrooz A, Jurkunas UV. Fuchs endothelial corneal dystrophy. Ocular Surf. 2010; 8(4):173–84.
External Disease and Cornea. Basic and Clinical Science Course, Section 8, 2011–2012. San Francisco: American Academy of Ophthalmology; 2011.
Friedberg M, Rapuano C. Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 5th ed. New York: Lippincott, Williams and Wilkins; 2008.
Hjortdal J, Pedersen IB, Bak-Nielsen S, Ivarsen A. Graft rejection and graft failure after penetrating keratoplasty or posterior lamellar keratoplasty for Fuchs endothelial dystrophy. Cornea. 2013;2(5): e60-e63.
Kanavi MR, Javadi MA, Sanagoo M. Indications for penetrating keratoplasty in Iran. Cornea. 2007;26(5):561–3.
Kang PC, Klintworth GK, Kim T, et al. Trends in the indications for penetrating keratoplasty, 1980–2001. Cornea. 2005;24(7):801–3.
Siganos CS, Tsiklis NS, Miltsakakis DG, et al. Changing indications for penetrating keratoplasty in Greece, 1982–2006: a multicenter study. Cornea. 2010;29(4):372–4.
Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore corneal transplant study. Ophthalmology. 2008;115(6):975–982.e1.
Wylegała E, Dobrowolski D, Tarnawska D, Wróblewska-Czajka E, Jurewicz A. [Indications for keratoplasty in District Railway ospital in Katowice]. Klin Oczna. 2005;107(10-12):646–9. Polish.