EPIDEMIOLOGY
Global Information
- Toxocariasis is one of the most prevalent zoonotic infections worldwide, but the majority of cases occur where dogs and cats (often household pets) are kept in close proximity to humans.
- Ocular toxocariasis is more prevalent in warmer climates because development of infectious Toxocara eggs occurs above 50°F (10°C).
- The seroprevalence of Toxocara infection has been estimated to be 4%–31% in developed countries. In tropical regions, where environmental conditions favor the transmission of geohelminths, it has been reported to be as high as 76% (Fan et al).
- Despite the high prevalence of Toxocara infections in some areas, little epidemiological information exists on the prevalence of ocular toxocariasis.
DIFFERENTIAL DIAGNOSIS
- Retinoblastoma (Figure 1)
- Traumatic, bacterial, fungal endophthalmitis (Figure 2)
- Coats disease (Figure 3)
- Familial exudative vitreoretinopathy
- Persistent fetal vasculature (PFV) (Figure 4)
- Other causes of pars planitis (Figure 5)
- Toxoplasmosis (Figure 6)
- Retinopathy of prematurity (ROP) (Figure 7)
- Combined hamartoma of the retina and retinal pigment epithelium (RPE)
- Diffuse unilateral subacute neuroretinitis (DUSN)
PATHOPHYSIOLOGY/DEFINITION
- Ocular toxocariasis is an uncommon disease that affects mostly children and young adults, resulting in significant vision loss. (Figure 8)
- It is caused by invasion of the second-stage larvae of two intestinal roundworms: Toxocara canis and Toxocara cati (a common parasite of dogs and cats, respectively).
- Humans acquire the infection by accidental ingestion of contaminated soil or food with Toxocara eggs, or the oral-fecal route; the roundworm parasites complete their life cycles in the small intestines entering circulation and migration to different organs.
Pathogenesis
- Disease can present as immediate and delayed hypersensitivity responses, depending on tissue type and host damage. (Figure 9)
- Liver, lungs, and central nervous system are commonly infected by visceral larvae migrans (VLM), and the eyes by ocular larvae migrans (OLM).
- In OLM, larvae migrate to the eye through: (1) the central retinal artery reaching the posterior pole, (2) the long ciliary arteries, or (3) the vitreous reaching the pars plana, producing inflammation and formation of granuloma.
- OLM is usually unilateral.
SIGNS/SYMPTOMS
Diagnosis of ocular toxocariasis is based on clinical findings (Table 1). OLM generally affects children between 2 and 10 years old, presenting as unilateral decreased visual acuity with or without strabismus. If clinical suspicion of ocular toxocariasis arises, the examiner should attempt to elicit a history of exposure to puppies and soil.
Characteristic findings:
- Pain
- Photophobia
- Decreased vision
- Floaters
- Leukocoria (late-stage disease) 25% of cases (Figure 10)
- Strabismus
- Posterior pole granuloma 25% of cases (Figure 11)
- Peripheral granuloma with or without traction bands 50% of cases (Figure 12)
- Pars planitis
- Vitritis
- Active chorioretinitis
- Retinal detachment
- Cataract (posterior subcapsular granuloma-like opacity)
|
Table 1. Clinical Presentations of Ocular Toxocariasis
|
|
Clinical
Syndrome
|
Age of Onset
|
Characteristic
Findings
|
|
Chronic endophthalmitis
|
2–9
|
Anterior uveitis and vitritis
Possible peripheral granuloma or grayish-white exudates near pars plana
|
|
Posterior pole granuloma
|
6–14
|
Round, yellow-white solid granuloma in macula or peripapillary region (Figure 13) as above
Minimal or absent intraocular inflammation
|
|
Peripheral granuloma
|
6–40
|
Peripheral granuloma/mass with dense connective tissue bands in the vitreous that extend from the lesion to the posterior pole. (Figure 14) as above
|
LABORATORY EVALUATION
- Enzyme-linked immunosorbent assay (ELISA)
- Serum titer of 1:8 is 91% sensitive and 90% specific for previous exposure
MANAGEMENT/TREATMENT
Medical and surgical therapies are used to treat this condition. The goal of treatment is foremost vision preservation, as the prognosis for visual recovery is typically guarded.
- Medical therapy
- First-line treatment: 5-day course of albendazole (10 mg/kg/day)
- Second-line treatment: Consider other benzimidazoles (mebendazole, tiabendazole, or diethylcarbamazine)
- Note: Ivermectin has not shown significant efficacy for the treatment of T canis or T cati
- Consider systemic or periocular corticosteroids to suppress the immune response and limit structural complications of the disease at a rate of 0.5 to 1 mg/kg/day
- If inflammation persists and the disease appears to be progressing, surgical therapy should be considered.
- Surgical therapy
- Pars plana vitrectomy
- No definitive protocols on treatment.
- Methods include all variations of vitrectomy (use of SF6 gas or silicon oil, with or without scleral buckle/lens extraction).
- Perfluorocarbon liquids injection
- Indicated to facilitate removal of epiretinal membranes (ERMs) and the posterior hyaloid in cases of tractional retinal detachment.
- Cryotherapy
- Applied directly at the areas of exudation at the pars plana with a double freeze-thaw technique.
- Endolaser
- Indicated for treatment of ocular granulomas
- Prognosis
- Visual acuity recovery is directly related to the anatomical location of the lesions, the degree of retinal detachment, and compromise of the photoreceptors, external limiting membrane, and optic nerve. Vision loss can therefore be variable, from mild to severe.
- Early treatment should be performed to decrease complications and provide better visual outcomes.
- Most cases of ocular toxocariasis have visual acuity of less than 20/40 at presentation.
- As in any pediatric condition that affects one eye, amblyopia treatment is an important component of the overall visual rehabilitation scheme.
CASE STUDY
Age
Gender
History of Present Illness
- Blurring of vision in right eye for 2 days prior to admission that has progressively worsened. She also complained of right eye discomfort, redness, and pain 1 day after the onset of illness.
- Denies any eye discharges, trauma, or foreign body from/to the right eye. There is a history of contact with neighborhood dogs. Denies history of fever, skin rashes, or upper respiratory tract infection symptoms. No previous history of eye diseases and no history of similar conditions among family members.
Physical Exam
- Vital signs stable and afebrile. Systemic examination was unremarkable except for palpable lymph nodes in the submandibular region.
Visual Exam
- Right eye: injected and nonreactive with corneal edema and mildly dilated pupils.
- Left eye: unremarkable.
- Ocular echography confirmed the presence of chronic endophthalmitis and exotropia of the right eye (Figure 15).
- Retinal exam demonstrated a temporal-peripapillary granuloma with tractional retinal detachment and dragged retina (Figure 16A).
Management/Treatment
- Toxocara canis IgG was confirmed in vitreous sample in a concentration of 1/256.
- Pars plana vitrectomy was performed with release of traction and improvement of the retinal anatomy (Figure 16B).
IMAGE LIBRARY
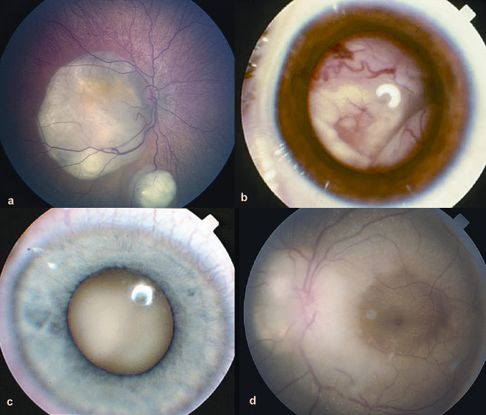
Figure 1. Retinoblastoma. (© 2013 American Academy of Ophthalmology)
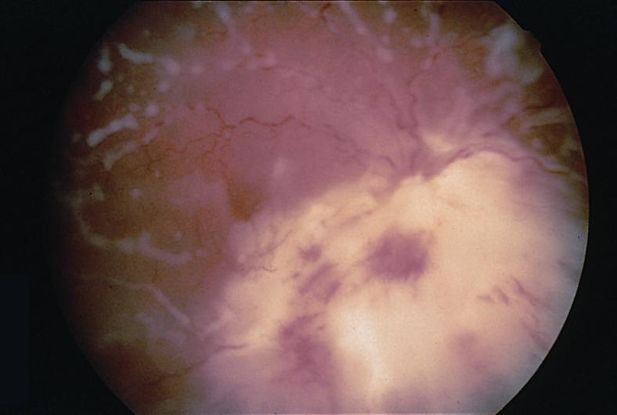
Figure 2. Endophthalmitis. (© 2013 American Academy of Ophthalmology)
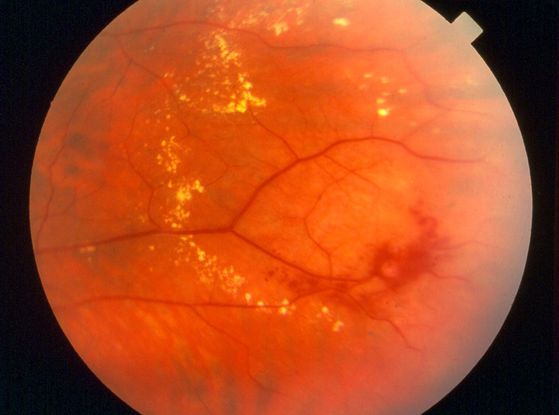
Figure 3. Coats disease. (© 2013 American Academy of Ophthalmology)
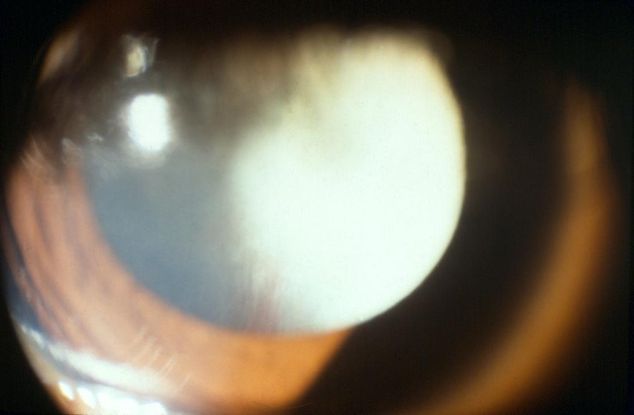
Figure 4 (© 2013 American Academy of Ophthalmology)
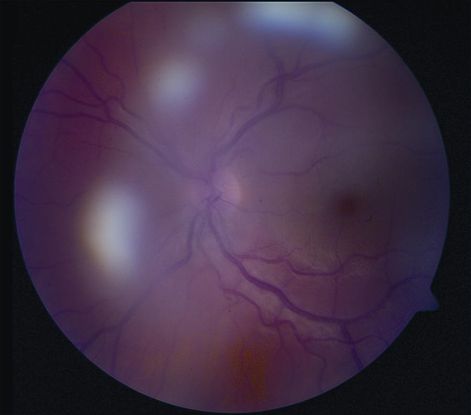
Figure 5. Pars planitis. (© 2013 American Academy of Ophthalmology)
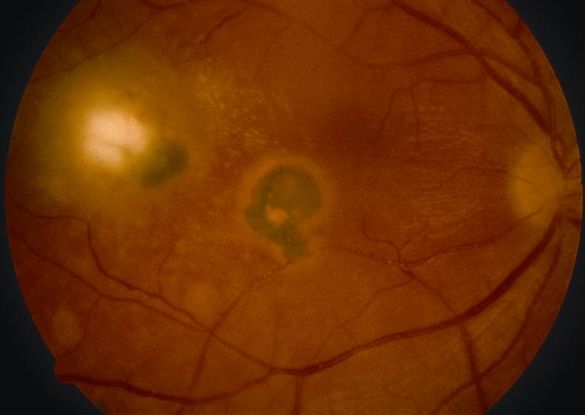
Figure 6. Toxoplasmosis. (© American Academy of Ophthalmology)
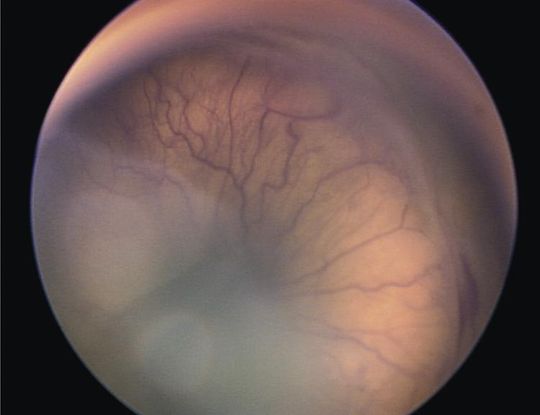
Figure 7. Retinopathy of Prematurity (ROP). (© 2013 American Academy of Ophthalmology)
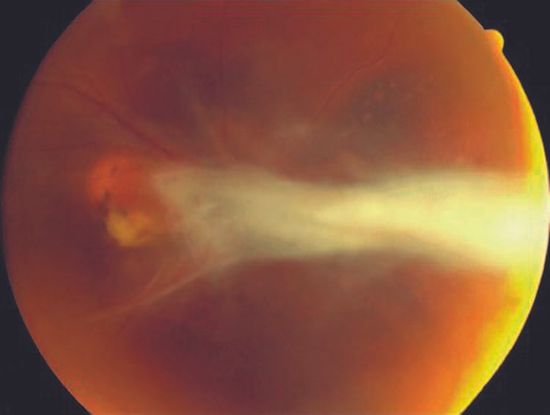
Figure 8. Ocular Toxocariasis. (© American Academy of Ophthalmology. Courtesy of Paul Griggs, MD.)
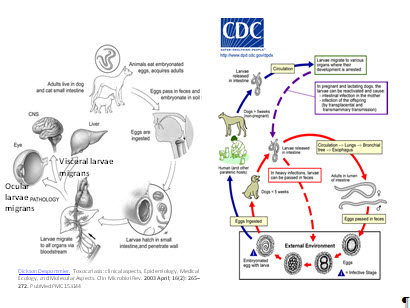
Figure 9. Toxocariasis pathogenesis and life cycle. (Left) Reproduced, with permission, from Clin Microbial Rev . 2003; 16(2): 265-272, and (right) Centers for Disease Control and Prevention (CDC), Atlanta, Georgia
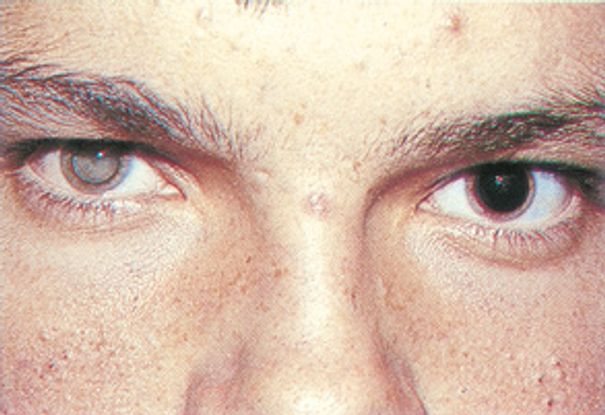
Figure 10. Toxocariasis: leukocoria. (© 2014 American academy of Ophthalmology)
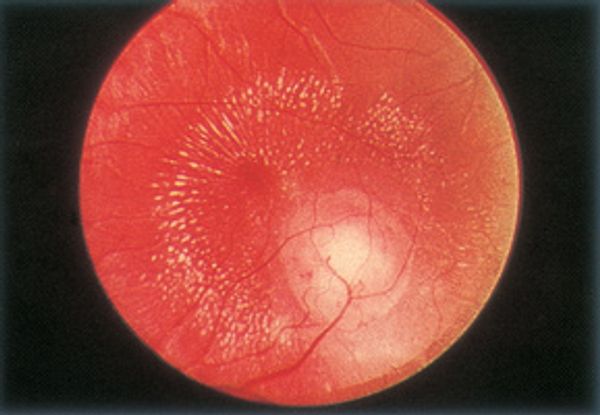
Figure 11. Toxocariasis: macular granuloma. (© 2014 American Academy of Ophthalmology)
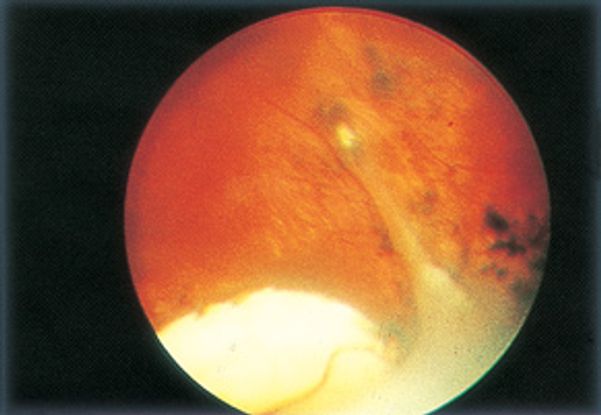
Figure 12. Toxocariasis: peripheral granuloma. (©2014 American Academy of Ophthalmology)
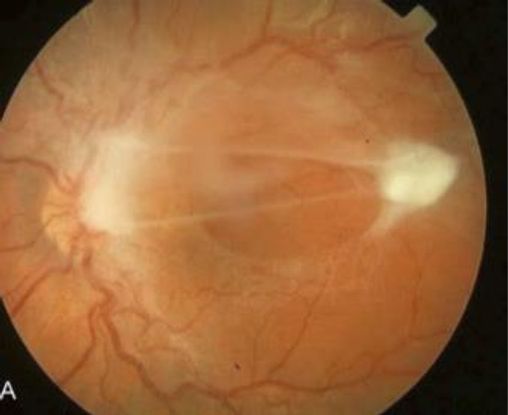
Figure 13. Toxocara posterior pole granuloma causing macular pucker and tractional retinal detachment in a 9-year-old girl; visual acuity 20/200. (© American Academy of Ophthalmology. Courtesy of Ramana S. Moorthy, MD.)
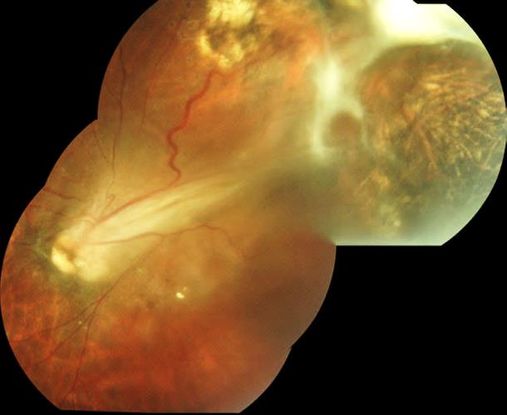
Figure 14. Superotemporal Toxocara peripheral granuloma of left eye with associated tractional retinal detachment and retinal fold through macula. (© 2014 American Academy of Ophthalmology. Courtesy of Ramana S. Moorthy, MD.)
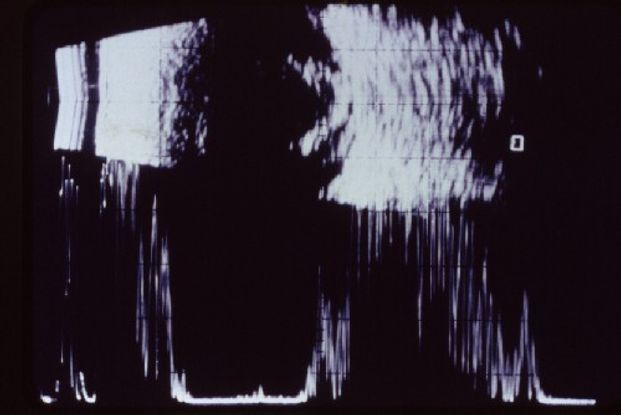
Figure 15. Chronic endophthalmitis seen in ocular echography. (© American Academy of Ophthalmology.)
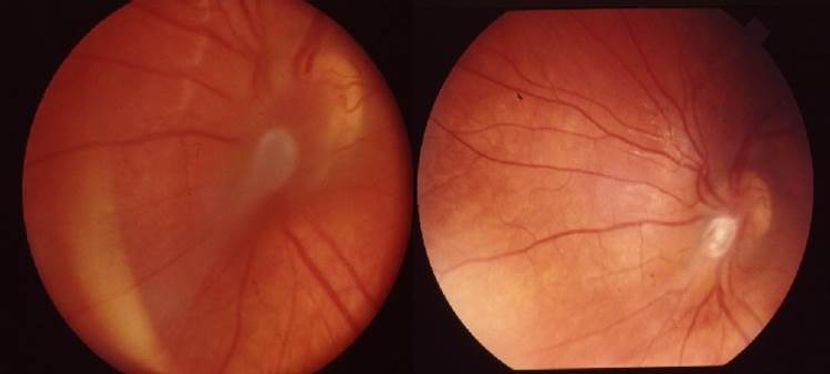
Figure 16A, 16B. Fundus photograph before (A) and after (B) pars plana vitrectomy. A. Temporal- peripapillary granuloma with tractional retinal detachment and dragged retina. B. Traction release and restoration of the retinal anatomy after pars plana vitrectomy. (© 2014 American Academy of Ophthalmology.)
REFERENCES
Agudelo C, Villareal E, Cáceres E, et al. Human and dogs Toxocara canis infection in a poor neighborhood in Bogota. Memórias do Instituto Oswaldo Cruz. 1990; 85:75–78.
Ahn SJ, Woo SJ, Hyon JY, Park KH. Cataract formation associated with ocular toxocariasis. J Cataract Refract Surg. 2013; 39(6):830-835.
Alabiad CR, Albini TA, Santos CI, Davis JL. Ocular Toxocariasis in a Seronegative Adult. Ophthalmic Surg Lasers Imaging (official journal of the International Society for Imaging in the Eye). 2010; April: 1–3. doi: 10.3928/15428877-20100325-06. [Epub ahead of print]
Amin HI, McDonald HR, Han DP, et al. Vitrectomy update for macular traction in ocular toxocariasis. Retina. 2000; 20(1): 80–85.
Archelli S, Kozubsky L. Toxocara y Toxocariosis. Acta Bioquím Clín Latinoam. 2008; 42(3): 379–384.
Arevalo JF, Espinoza JV, Arevalo FA. Ocular toxocariasis. J Pediatr Ophthalmol Strabismus. 2013; 50(2): 76–86.
Arevalo JF, Garcia-Amaris RA. The role of vitreo-retinal surgery in children with uveitis. Intl Ophthalmol Clin. 2008; 48(3): 153–172.
Caumes E. Treatment of cutaneous larva migrans and Toxocara infection. Fundam Clin Pharmacol. 2003; 17(2): 213–216.
Chieffi PP, Santos SV, Queiroz ML, Lescano SA. Human toxocariasis: contribution by Brazilian researchers. Rev Inst Med Trop São Paulo. 2009; 51(6): 301–308.
Delgado O, Rodríguez-Morales AJ. Aspectos clínico- epidemiológicos de la toxocariasis: una enfermedad desatendida en Venezuela y América Latina. Boletín de malariología y salud ambiental. 2009; 49(1): 1–33.
Despommier D. Toxocariasis: Clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16(2): 265- 272.
De Visser L, Rothova A, de Boer JH, et al. Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am J Ophthalmol. 2008; 145(2): 369–374.
Espinoza YA, Huapaya PH, Roldán WH, Jiménez S, Arce Z, Lopez E. Clinical and serological evidence of Toxocara infection in school children from Morrope District, Lambayeque, Peru. RevInst Med Trop São Paulo. 2008; 50(2): 101–105.
Fan CK, Hung CC, Du WY, Liao CW, Su KE. Seroepidemiology of Toxocara canis infection among mountain aboriginal schoolchildren living in contaminated districts in eastern Taiwan. Trop Med Int Health. 2004; 9:1312-1318.
Nava Cortéz N, Romero Nuñez C, Bautista Gómez LG, Hernández García PA, Heredia Cárdenas R. Presence of anti-Toxocara canis antibodies and risk factors in children from the Amecameca and Chalco regions of México BMC Pediatr. 2015; 15: 65.
Pollack AL, McDonald HR, Johnson RN, et al. Peripheral retinoschisis and exudative retinal detachment in pars planitis. Retina. 2002; 22(6) :719–724.
Rodriguez A. Early pars plana vitrectomy in chronic endophthalmitis of toxocariasis. Graefes Arch Clin Exp Ophthalmol.1986; 224: 218–220.
Rodriguez Gonzalez A. Vitrectomia en la uveitis. Medicina (Bogota´). 1989; 20: 6–12.
Roldán WH, Cavero YA, Espinoza YA, Jiménez S, Gutiérrez CA. Human toxocariasis: a seroepidemiological survey in the Amazonian city of Yurimaguas, Peru. Rev Inst Med Trop São Paulo. 2010; 52 (1): 37–42.
Roldán WH, Espinoza YA, Huapaya PE, Huiza AF, Sevilla CR, Jiménez S. Frequency of human toxocariasis in a rural population from Cajamarca, Peru, determined by DOT-ELISA test. Rev Inst Med Trop São Paulo. 2009; 51(2): 67–71.
Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010; 104(1): 3–23.
Shields JA. Ocular toxocariasis: A review. Surv Ophthalmol. 1984; 28(5): 361-381.
Stewart JM, Cubillan LD, Cunningham ET Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005; 25 (8): 1005–1013.
Werner JC, Ross RD, Green WR, Watts JC. Pars plana vitrectomy and subretinal surgery for ocular toxocariasis. Arch Ophthalmol. 1999; 117(4): 532–534.
Woodhall D, Starr MC, Montgomery SP, et al. Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology. 2012;119(6):
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editor:
Asia-Pacific:
Timothy Y. Lai, MBBS, MD, MMedSc, FRCS, FRCOphth, FHKAM, Department of Ophthalmology & Visual Sciences, The Chinese University of Hong Kong
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2014 American Academy of Ophthalmology®. All Rights Reserved.