EPIDEMIOLOGY
Global Information
- Polypoidal choroidal vasculopathy (PCV) primarily affects pigmented individuals, especially Asians and African-Americans
- Typically presents in 7th to 8th decade of life, though may present earlier than other forms of macular degeneration
- Caucasians are affected to a lesser extent and with different clinical features
- Table 1 highlights the observed differences in PCV characteristics across ethnicities.
Table 1: Polypoidal Choroidal Vasculopathy Demographics and Characteristics |
|
Ethnicity
|
Mean Age
|
% Male
|
% Bilateral
|
% Central Macula Involving
|
|
White
|
60-70
|
<50
|
20-80
|
25-68
|
|
Japanese
|
60-70
|
>60
|
<10
|
>85
|
Regional Information (Europe)
- 75% female
- Primarily peripapillary
- 32% bilateral
- In Caucasian populations, the disease accounts for 4-13% of presumed exudative AMD cases, including:
- 8.2% in Greek
- 9.8% in Italian
- 13.9% in German patients. Indeed, the prevalence of IPCV reaches 85% among patients presenting with large hemorrhagic and exudative neurosensory detachments. In Chinese patients, studies revealed a percentage between 22.3% and 24.5% affected with IPCV, among patients with exudative AMD. Reported frequencies of IPCV in Japanese patients vary between 23% and 54.7% of those initially diagnosed with exudative AMD, while in Korean patients, this percentage reaches 24.6%
DIFFERENTIAL DIAGNOSIS
- AMD
- Choroidal neovascularization (CNV)
- Central serous chorioretinopathy (CSC)
- Pathologic myopia
- Melanocytoma
- Choroidal Tumors (hemangioma, melanoma, etc)
- Tilted disc syndrome
- Choroidal osteoma
PATHOPHYSIOLOGY/DEFINITION
PCV is a neovascular and hemorrhagic disorder of the choroid.
- Degeneration of the walls of arterioles, capillaries, and venules
- Large vascular channels and “polypoid” formations
- Thickened capillary basement membranes
- Hyalinization of choroidal vessels
- Exudation of fibrin and plasma
Genetic Factors
- Gene HTRA1 is associated with PCV as well as neovascular AMD
- Complement factor H (CFH) variants rs3753394 and rs 800292 are associated with PCV
- CRP levels are higher in PCV patients and in AMD patients than in control patients
- I62V variant of CFH is associated with PCV in Japanese patients
- ARMS2 gene for mitochondrial photoreceptor protein is associated with PCV in Japanese patients
Vascular endothelial growth factor (VEGF) plays an uncertain role in the pathophysiology of PCV.
- VEGF has been localized to the retinal pigment epithelium (RPE) in patients with PCV
- VEGF has not been seen in the vascular endothelium on immunostaining of surgical specimens
- Anti-VEGF treatment has been well-reported to be effective in treating fluid associated with PCV; however, it has shown inconsistent results in resolving the polypoid vascular lesions
Clinical Course
1. Can be variable in severity, ranging from spontaneous resolution to recurrent subretinal bleeding and subretinal pigment epithelial bleeding
2. Recurrent serosanguinous detachments of the RPE or neurosensory retina
3. Microtears of RPE
4. RPE atrophy
5. Vision loss in ~ 50% of patients
SIGNS/SYMPTOMS
Clinical Characteristics
- Serosanguinous detachments of the RPE and neurosensory retina
- PCV lesions and choroidal neovascularization (CNV)
- Typically peripapillary or central macula location
- Relatively preserved retinal architecture
- Better-than-expected visual acuity
- Pigment epithelial detachment associated with polypoid lesions
- Chronic lipid deposition
- Occasional spontaneous resolution with residual pigment mottling
Clinical characteristics may differ in Caucasian patients;
- Lesions are primarily peripapillary
Imaging
Fluorescein Angiogram (FA)
- Branching choroidal vessels
- Polypoidal lesions can at times be difficult to see on FA. Lesions may manifest as either classic or occult leakage patterns
- Atrophy of RPE
Indocyanine Green Angiography (ICG)
- Choroidal vessel hyperpermeability
- Pulsatile polypoidal vessels
- Choroidal vascular networks
Optical Coherence Tomography (OCT)
- Sub-RPE polypoidal lesions
- Pigment epithelial detachments
MANAGEMENT (Europe)
Treatment is offered to patients with persistent or progressive exudative manifestations of the disease.
Medical therapy
- Anti-VEGF monotherapy with ranibizumab or bevacizumab has led to improved visual acuity in patients with IPCV; however, the visual outcome with treatment is usually inferior to that seen in patients with exudative AMD and frequent retreatments are necessary.
- Photodynamic therapy (PDT) with verterporfin in subfoveal polypoidal lesions has shown more encouraging results in both white and pigmented patients
- This has been supported in several studies in European patients
- A study of Portuguese patients with 3-year follow-up:
- 74.1% of patients showed improved or stable visual acuity
- 14.8% of patients showed significant improvement in visual acuity despite a high recurrence rate.
- A study of Greek patients compared PDT monotherapy, ranibizumab monotherapy, and combined therapy with PDT and ranibizumab,
- PDT monotherapy was superior to the other treatment modalities in terms of improvement in visual acuity.
- In a relevant comparative study in Asian patients, PDT monotherapy and combined therapy with PDT and anti-VEGF have been advocated as superior to anti-VEGF monotherapy.
Medical Follow-up
Patients are followed every 4-6 weeks when receiving intravitreal injections, whereas in laser treated eyes the intervals can be longer (every 3 months).
Surgery
Surgical intervention is rarely advocated. Pars plana vitrectomy is indicated in cases of subretinal hemorrhage with breakthrough bleeding in the vitreous. Pneumatic displacement of subretinal hemorrhage and tissue plasminogen activator-assisted removal of subretinal blood have also been suggested with variable results, but are not routinely performed.
Surgical Follow-up
Follow-up in these cases is the same as for pars plana vitrectomy for other conditions. Medical treatment with laser or anti-VEGF agents can implement surgery.
Complications
Adverse events in anti-VEGF treated eyes include anterior chamber inflammation, progression of cataract, macular hole, and new subretinal or vitreous hemorrhage, especially in cases with larger PCV lesions. In addition, changes in the pattern of PCV after PDT, resulting in persistent or recurrent exudation, have been reported.
Prognosis
Since the natural history of the disease is generally good in 50% of patients, observation is often initially indicated. Spontaneous autoinfarction of the polyps with flattening of the retinal pigment epithelium and clearing of the hemorrhage have been reported. However, severe visual loss can result from macular degeneration and atrophy and (rarely) even from global detachments and rubeosis iridis. Initial visual acuity, location and size of the lesion are factors that affect the prognosis with treatment.
CASE IMAGES: EUROPE
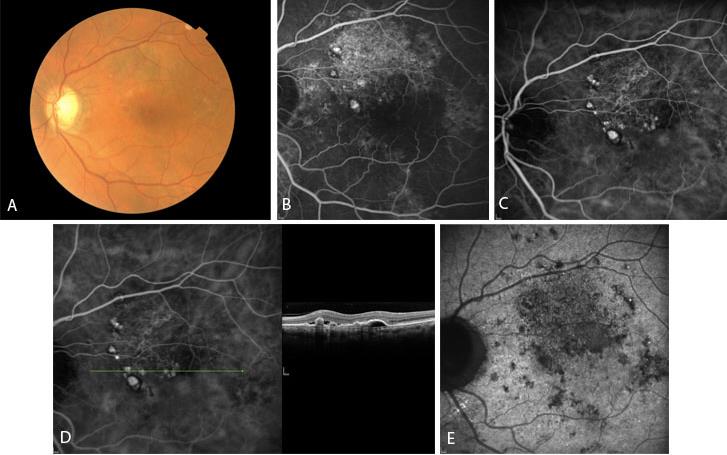
Figure 1 Case Images Europe. A 64- year-old man with reduced visual acuity for 1 month. BCVA: 20/70. Reddish round structure nasally to the fovea in color photograph (A) represents polyp that is revealed in fluorescein angiography (B) and in indocyanine green angiography (C). More polyps are obvious in ICG. Section through the polyps in OCT (D) reveals local pigment epithelial detachments. See also the optical attenuation under the polyp. In very late phase of indocyanine green (E) the characteristic “wash-out” of the polyps is seen. (Case and images courtesy of Alexandros Rouvas MD, Attikon University Hospital, Athens, Greece.)
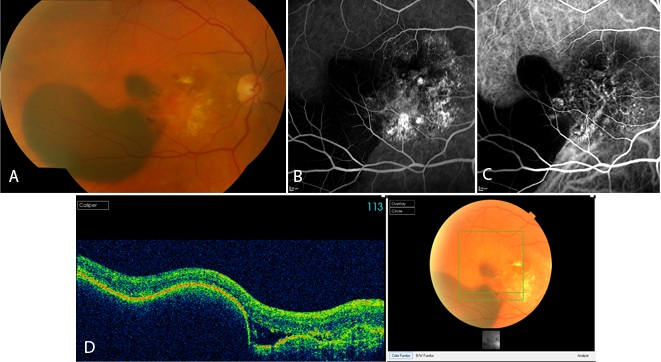
Figure 2 Case Images Europe. A 60-year-old woman with reduced vision for 20 days. BCVA: 20/100. Extensive hemorrhage temporal to the fovea in combination with lipid exudates in the central macula is seen in the color photograph (A). Fluorescein angiography (B) and indocyanine green (C) reveal polypoidal lesions. Optical coherence tomography sections through the hemorrhage reveals retinal pigment epithelial detachment and subretinal fluid (D). (Case and images courtesy of Alexandros Rouvas MD, Attikon University Hospital, Athens, Greece.)
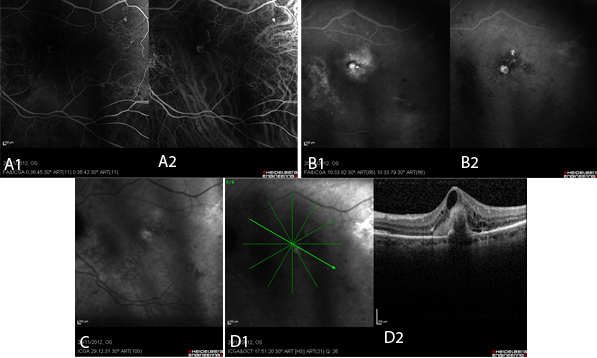
Figure 3 Case Images Europe. Polypoid lesions in combined simultaneous fluorescein and indocyanine green angiography. A: Early phase: Polyps appear as well-demarcated hyperfluorescent areas in both fluorescein (A1) and indocyanine (A2) images.
B: Late phase: In FA (B1), the polyp appears hyperfluorescent surrounded by diffuse hyperfluorescence. In ICG (B2) the polyps are clearly present as hyperfluorescent structures. C: In very late phase of ICG, the characteristic “wash-out” phenomenon leads to hypofluorescence within the polyp accompanied by hyperfluorescence of its borders. D: OCT of the same patient; section through the polyp (D1). Notice the attenuation of the optical signal under the area of the polyp (D2). (Images courtesy of Alexandros Rouvas MD, Attikon University Hospital, Athens, Greece,)
REFERENCES
Ahuja RM, Stanga PE, Vingerling JR, et al. Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelium detachments. Br J Ophthalmol. 2000; 84(5): 479-484.
Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148 (1): 70-78.
Gemmy Cheung CM, Yeo I, Li X et al. Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol . 2013; 155: 295-304 e291.
Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for choroidal vasculopathy. Br J Ophthalmol. 2008; 92(1): 70-73.
Imamura, et al. Polypoidal Choroidal Vasculopathy: A Review. Surv Ophthalmol. 2010: 55(6).
Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment naïve patients with polypoidal choroidal vasculopathy. Retina. Accessed July 18, 2014, Epub ahead of print.
Jeon S, Lee WK and Kim KS. Adjusted retreatment of polypoidal choroidal vasculopathy after combination therapy: results at 3 years. Retina. 2013; 33: 1193-1200.
Kondo N, Honda S, Ishibashi K, et al. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007; 144(4): 608-612.
Kwok AK, Lai TY, Chan CW, et al. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002; 86(6): 892-897.
Lafaut BA, Leys AM, Snyers R, et al. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000; 238(9): 751-759.
Lee KY, Vithana EN, Mathur R, et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(6): 2613-2619.
Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye. 2009; 23(1):145-148.
Maruko I, Iida T, Saito M, et al. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144(1): 15-22.
Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinico-pathological findings of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(11): 4729-4737.
Okubo A, Sameshima M, Uemura A, et al. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002; 86(10): 1093-1098.
Pantaleoni FB, Galante VM, Da Dalt S, Pecorella I. Localizing polypoidal choroidal vasculopathy for laser treatment. Acta Ophthalmol Scand. 2007; 85(4): 456-458.
Reche-Frutos J, Calvo-Gonzales C, Donate-Lopez J, et al. Short-term anatomic effect of ranibizumab for polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2008; 18(4): 645-648.
Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. Accessed July 30, 2014, Epub ahead of print.
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121: 1392-1396.
Spaide RF, Yannuzzi LA, Slakter JS, et al. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15: 100-110.
Terasaki H, Miyake Y, Suzuki T, et al. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002; 86(3): 321-327.
Tsujikawa A, Sasahara M, Otani A, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007; 143(1): 102-111.
Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47(4): 379-384.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editor:
Europe:
Christiane Isolde Falkner-Radler, MD, The Ludwig Boltzmann Institute of Retinology and Biomicroscopic Laser Surgery; Department of Ophthalmology, Rudolf Foundation Clinic
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
Regional Contributors:
Europe:
Miltiadis K Tsilimbaris, MD, PhD, Anna Dasteridou, MD, MSc, Department of Ophthalmology, University of Crete Medical School, Greece
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2014 American Academy of Ophthalmology®. All Rights Reserved.