EPIDEMIOLOGY
Global Information
- Polypoidal choroidal vasculopathy (PCV) primarily affects pigmented individuals, especially Asians and African-Americans; however, it is now also recognized in the white population.
- Typically presents in 6th to 7th decade of life, though may present earlier than other forms of macular degeneration
- Whites are affected to a lesser extent and with different clinical features
- Table 1 highlights the observed differences in PCV characteristics across ethnicities.
Table 1: Polypoidal Choroidal Vasculopathy Demographics and Characteristics |
|
Ethnicity
|
Mean Age
|
% Male
|
% Bilateral
|
% Peripapillary Involving
|
|
White
|
60-70
|
<50
|
20-80
|
35-75
|
|
Asian
|
60-70
|
>60
|
<10
|
<15
|
Regional Information (Africa)
- In a 5-year review among patients attending the Retina Clinic of the University College Hospital Ibadan, Nigeria
- 10 cases of PCV
- Mean age: 58 years
- 25% Male
DIFFERENTIAL DIAGNOSIS
RISK FACTORS/BIOMARKER
- Shared risk factors with AMD-CNV
- Age, male gender, smoking, hypertension, coronary artery disease, and hyperlipidemia
- Female gender was found to be a protective factor
- Large study in Korea: Age, body mass index (BMI), and higher education to be more strongly associated with PCV than AMD-CNV
- Ocular risk factors
- A history of CSC
- Systemic biomarkers
- C-reactive protein (CRP)
- Homocysteine
- Matrix metalloproteinase (MMP)
- Aqueous/vitreous biomarkers
- a. Interleukin-1b (IL-1b)
- VEGF
- Pigment epithelium derived factor (PEDF)
PATHOPHYSIOLOGY/DEFINITION
PCV is a neovascular and hemorrhagic disorder of the choroid.
Histology
- Degeneration of the walls of arterioles, capillaries, and large, dilated venules
- Large vascular channels and “polypoid” formations (Figure 9)
- Thickened capillary basement membranes
- Hyalinization of choroidal vessels
- Exudation of fibrin and plasma
Genetic Factors
- Gene HTRA1 is associated with PCV as well as neovascular AMD
- Complement factor H (CFH) variants rs3753394 and rs 800292 are associated with PCV
- CRP levels are higher in PCV patients and in AMD patients than in control patients
- I62V variant of CFH is associated with PCV in Japanese patients
- ARMS2 gene for mitochondrial photoreceptor protein is associated with PCV in Japanese patients
- CETP genetic variants to be associated with a high risk of PCV and is concordant with reports of elevated HDL as a risk factor for AMD, suggesting possible involvement of the high-density lipoprotein pathway
- Recent meta-analysis (2015)
- Complement cascade (CFH Y402H SNP rs1061170, CFH I62V SNP rs800292, C2 SNP rs547154, CFB SNP rs4151657, RDBP SNP rs3880457, and SKIV2L SNPs rs2075702 and rs429608)
- Inflammatory pathway (TNFRSF10A-LOC389641SNP rs13278062 and BEST-C4orf14-POLR2B-IGFBP7 SNP rs1713985)
- Extracellular matrix/basement membrane regulation pathway (ARMS2 A69S SNP rs10490924 and HTRA1 promoter SNP rs11200638) and lipid metabolism pathway (CETP SNP rs3764261)
- Previously associated with PCV (ARMS2, HTRA1, C2, CFB, ELN, LIPC, LPL, ABCA1, VEGF-A, TLR3, LOXL1, SERPING1, and PEDF) were not significantly associated with PCV in this meta-analysis
- Genotype associated with retreatment response
- ARMS2 LOC387715 rs10490924 variant: larger lesion size, higher likelihood of vitreous hemorrhage, and worse visual outcome after PDT or combination therapy
- ARMS2 A69S risk genotype :higher risk for second eye involvement
- CFH I62V polymorphism: associated with choroidal thickness
- ARMS2 A69S (rs10490924), HTRA1-rs11200638, PEDF gene polymorphism (SERPINF1 rs12603825): poor outcome after PDT
Vascular endothelial growth factor (VEGF) plays an uncertain role in the pathophysiology of PCV.
- VEGF has been localized to the retinal pigment epithelium (RPE) in patients with PCV
- Aqueous levels of VEGF were higher in PCV eyes compared to controls, but were significantly lower than aqueous VEGF levels in CNV-AMD
- VEGF has not been seen in the vascular endothelium on immunostaining of surgical specimens
- Anti-VEGF treatment has been well-reported to be effective in treating fluid associated with PCV; however, it has shown inconsistent results in resolving the polypoid vascular lesions
- While VEGF related angiogenesis processes were important in PCV pathogenesis, different angiogenesis pathways and cascades may be implicated as well
Natural Course
- Depends on factors including location (peripapillary vs macular), size of the lesion, and associated bleeding and exudation
- Polypoidal structures may also involute spontaneously
- Recurrent serosanguinous detachments of the RPE or neurosensory retina
- Microtears of RPE
- RPE atrophy
- Favorable course was seen in half of all patients with PCV while the other half had recurrences and eventual visual loss.
- 11% cumulative incidence of second-eye involvement in PCV in five years
Definition
- The Japanese Study Group of PCV
- Definite PCV: protruded orange-red elevated lesions on fundus examination and/or characteristic polypoidal lesions on ICGA
- Probable PCV: only an abnormal vascular network or occurrence of recurrent hemorrhagic and/or serous detachment of RPE were observed
- EVEREST study
- Subretinal focal ICGA hyperfluorescence with presence of at least one of the following
- Branching vascular networks (BVNs)
- Pulsatile polyp
- Nodular appearance on stereoscopic viewing
- Hypofluorescent halo
- Orange subretinal nodule on fundus photo
- Presence of massive submacular hemorrhage
SIGNS/SYMPTOMS
Clinical Characteristics
- Serosanguinous detachments of the RPE and neurosensory retina
- PCV lesions and choroidal neovascularization (CNV) (→The hallmark of PCV is the polypoidal lesion, which may be visible as an orange-red nodule on fundus examination)
- Typically peripapillary or central macula location
- Relatively preserved retinal architecture
- Better-than-expected visual acuity
- Pigment epithelial detachment associated with polypoid lesions
- Chronic lipid deposition
- Occasional spontaneous resolution with residual pigment mottling
- 2 types of PCV
- Exudative, characterized by serous PED and retinal detachment
- Hemorrhage, characterized by hemorrhagic PED and subretinal hemorrhage at the macula
Clinical characteristics may differ in white patients;
- Lesions are primarily peripapillary
- Drusen may also be seen in white patients
Imaging
Fluorescein angiogram (FA) (Figure 10)
- Branching vascular networks are often located in Bruch’s membrane (occult CNV)
- Polypoidal lesions can at times be difficult to see on FA. Lesions may manifest as either classic or occult leakage patterns
- Atrophy of RPE
Indocyanine green angiography (ICG) (Figure 11)
- Gold standard for diagnosis of PCV
- Choroidal vessel hyperpermeability
- Pulsatile polypoidal vessels
- Choroidal vascular networks
Optical coherence tomography (OCT) (Figure 12)
- Sub-RPE polypoidal lesions
- Pigment epithelial detachments
- Notch in the margin of large PED indicates the site of polypoidal lesions
- “Double layer sign”: two hyper-reflective lines represent separation of the RPE from Bruch’s membrane
- Higher rates of choroidal thickening
Fundus autofluorescence (FAF)
- Central hypo-autofluorescence with a circumferential hyper-autofluorescent ring corresponding to polypoidal lesions
- Granular hyperautofluorescence, corresponding to the branching choroidal vascular network
- Useful, non-invasive adjunct to ICGA and OCT for diagnosis and monitoring of treatment
OCT angiography
- The combination of en face and cross-sectional OCT angiographic images provides anatomical information about polypoidal structures
- More detailed view of type 1 CNV or branching vascular network complicated with PCV than ICGA
- Detection rate of polypoidal structures is lower than ICGA
- Only 50% of polyps were detected by OCT angiography from recent study performed in Korea
MANAGEMENT (Africa)
- Observation for extrafoveal lesions.
- Thermal laser for extrafoveal lesions threatening the fovea.
- PDT is not available here, and patients are often referred abroad for this treatment
- Vitrectomy for breakthrough vitreous hemorrhage
IMAGE LIBRARY
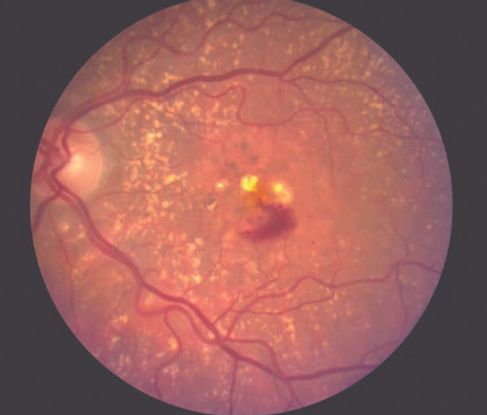
Figure 1. Age-related macular degeneration (AMD) ©American Academy of Ophthalmology, 2014.
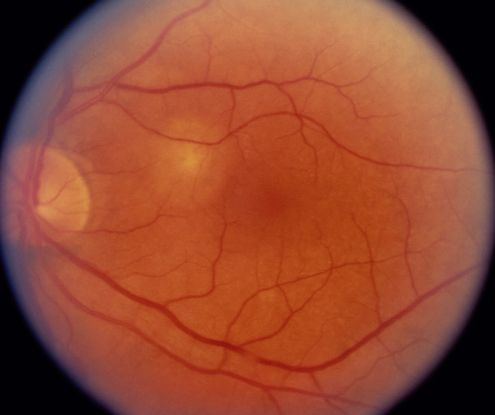
Figure 2. Choroidal neovascularization (CNV) ©American Academy of Ophthalmology, 2014.
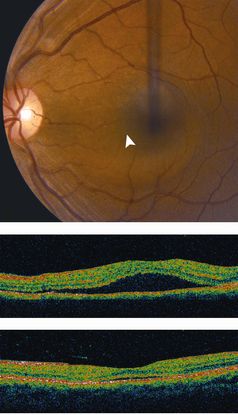
Figure 3. Central serous chorioretinopathy (CSC) ©American Academy of Ophthalmology, 2014.
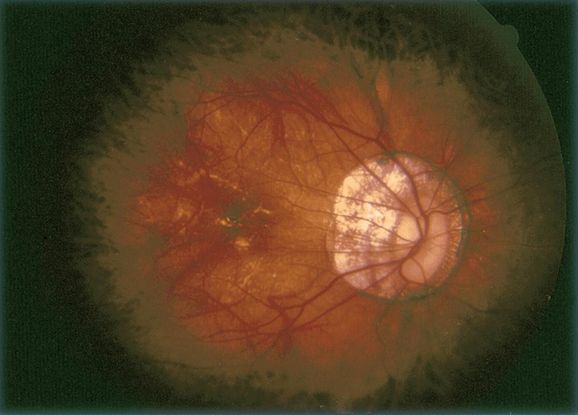
Figure 4. Pathologic myopia ©American Academy of Ophthalmology, 2014.
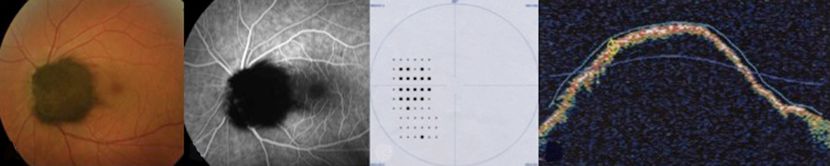
Figure 5. Melanocytoma ©American Academy of Ophthalmology, 2014.
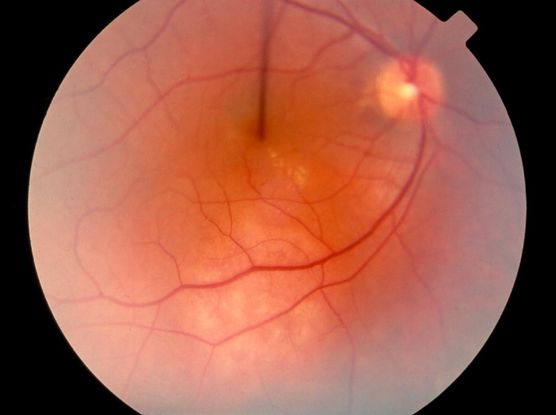
Figure 6. Choroidal tumors (hemangioma, melanoma, etc) ©American Academy of Ophthalmology, 2014.
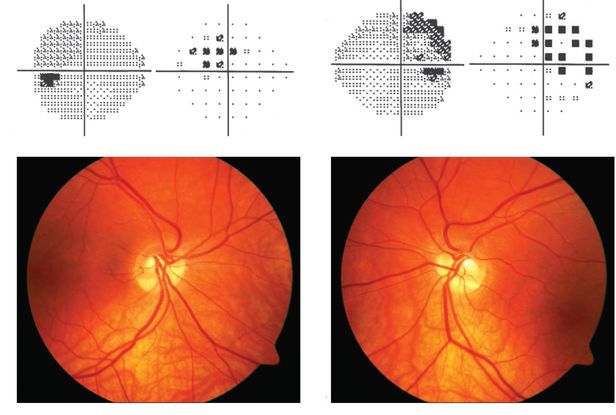
Figure 7. Tilted disc syndrome ©American Academy of Ophthalmology, 2014.
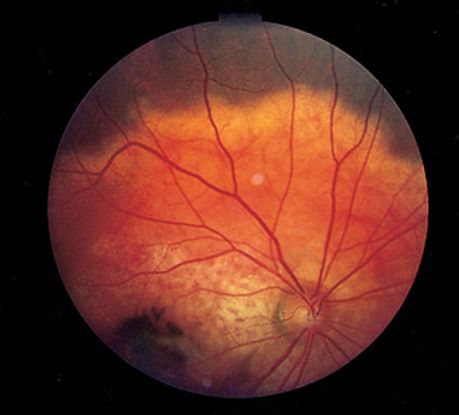
Figure 8. Choroidal osteoma ©American Academy of Ophthalmology, 2014.
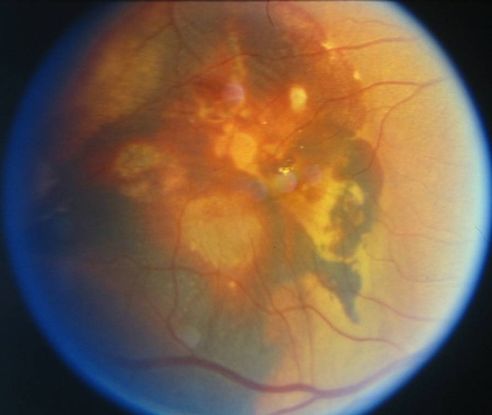
Figure 9. Large vascular channels and “polypoid” formations ©American Academy of Ophthalmology, 2014.
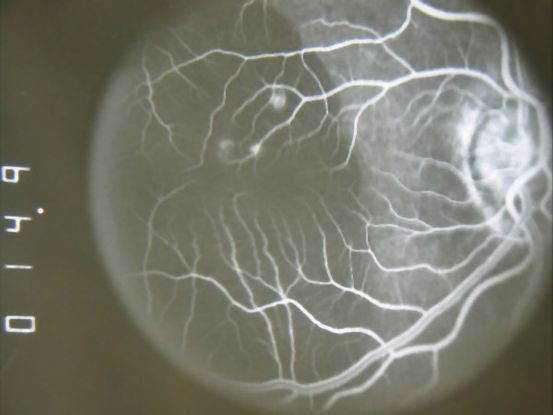
Figure 10. Fluorescein angiogram (FA) ©American Academy of Ophthalmology, 2014.
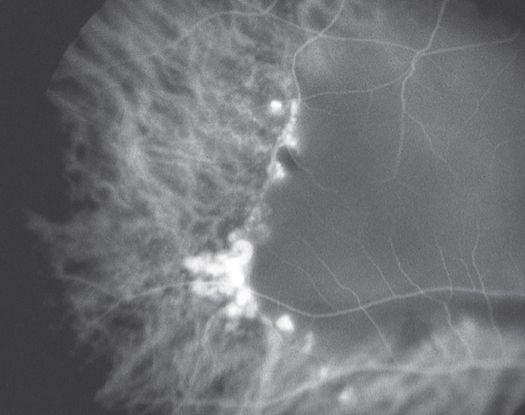
Figure 11. Indocyanine green angiography (ICG) ©American Academy of Ophthalmology, 2014.
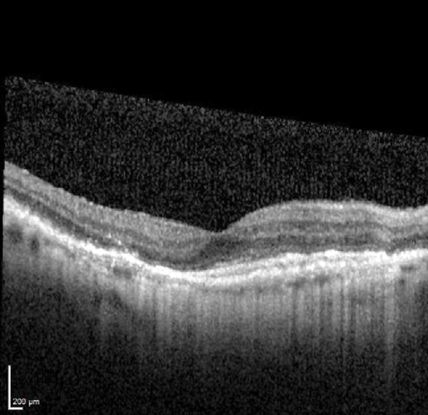
Figure 12. Optical coherence tomography (OCT) ©American Academy of Ophthalmology, 2014.
CASE IMAGES: Africa
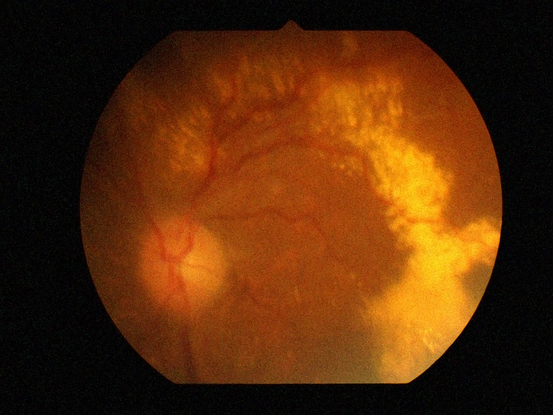
Figure 1 Case Images Africa. IPCV with chronic subretinal exudation in a 68-year-old Nigerian man.
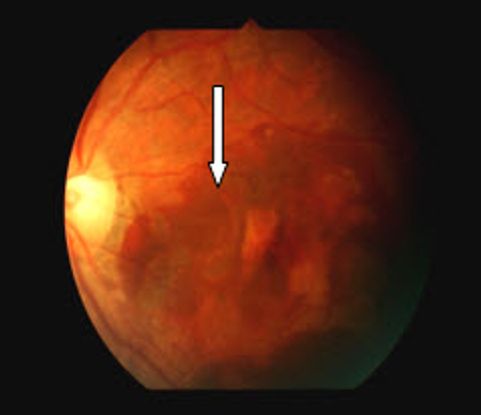
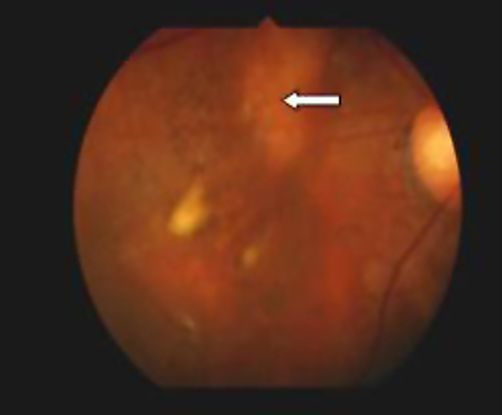
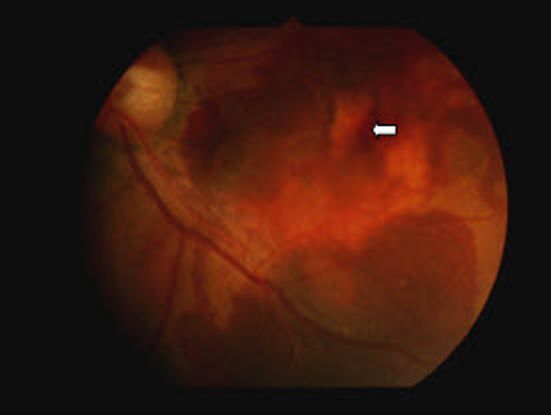
Figure 2 Case Images Africa. Fundus findings in a 56-year-old Nigerian woman with IPCV. Right eye with resolved subretinal blood and macular degeneration. The left eye shows fresh subretinal hemorrhage (long arrow); subretinal lesions (medium arrow); RPE detachment (short arrow). Note the absence of drusen seen in age-related macular degeneration.
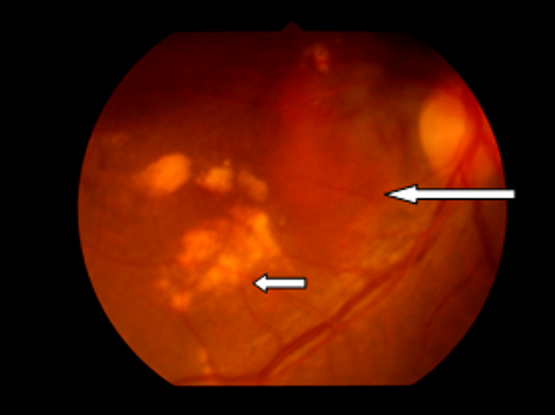
Figure 3 Case Images Africa. 53-year-old Nigerian woman with IPCV. Polypoidal, orange subretinal lesion (long arrow) and sub retinal exudates (short arrow)
REFERENCES
Ahuja RM, Stanga PE, Vingerling JR, Reck AC, Bird AC. Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelium detachments. Br J Ophthalmol. 2000; 84(5): 479-484.
Bozzoni Pantaleoni F, Magliari Galante V, Da Dalt S, Pecorella I. Localizing polypoidal choroidal vasculopathy for laser treatment. Acta Ophthalmol Scand. 2007; 85(4): 456-458.
Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148 (1): 70-78.
Gemmy Cheung CM, Yeo I, Li X, et al. Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol . 2013; 155: 295-304 e291.
Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for choroidal vasculopathy. Br J Ophthalmol. 2008; 92(1): 70-73.
ImamuraY, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal Choroidal Vasculopathy: A Review. Surv Ophthalmol. 2010: 55(6):501-515.
Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment naïve patients with polypoidal choroidal vasculopathy. Retina. 2014; 34(11):2178-2184.
Jeon S, Lee WK, Kim KS. Adjusted retreatment of polypoidal choroidal vasculopathy after combination therapy: results at 3 years. Retina. 2013; 33: 1193-1200.
Kondo N, Honda S, Ishibashi K, Tsukahara Y, Negi A. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol. 2007; 144(4): 608-612.
Kwok AK, Lai TY, Chan CW, Neoh EL, Lam DS. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002; 86(6): 892-897.
Lafaut BA, Leys AM, Snyers R, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000; 238(9): 751-759.
Lee KY, Vithana EN, Mathur R, et al. Association analysis of CFH, C2, BF, and HTRA1 gene polymorphisms in Chinese patients with polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(6): 2613-2619.
Lee MW, Yeo I, Wong D, Ang CL. Argon laser photocoagulation for the treatment of polypoidal choroidal vasculopathy. Eye (Lond.). 2009; 23(1):145-148.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007; 144(1): 15-22.
Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinico-pathological findings of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2008; 49(11): 4729-4737.
Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol. 2002; 86(10): 1093-1098.
Reche-Frutos J, Calvo-Gonzales C, Donate-Lopez J, Garcia-Feijoo J, Leila M, Garcia-Sanchez J. Short-term anatomic effect of ranibizumab for polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2008; 18(4): 645-648.
Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014; 34(11):2192-2201,
Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol. 2003; 121: 1392-1396.
Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995; 15: 100-110.
Terasaki H, Miyake Y, Suzuki T, Nakamura M, Nagasaka T. Polypoidal choroidal vasculopathy treated with macular translocation: clinical pathological correlation. Br J Ophthalmol. 2002; 86(3): 321-327.
Tsujikawa A, Sasahara M, Otani A, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2007; 143(1): 102-111.
Yuzawa M, Mori R, Haruyama M. A study of laser photocoagulation for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2003; 47(4): 379-384.
CONTRIBUTORS
Executive Editor:
R. V. Paul Chan, MD, FACS, Weill Cornell Medical College, New York, New York
Section Editor:
Sub-Saharan Africa:
Dupe Ademola-Popoola, MBBS, FMCOphth, FWACS, Department of Ophthalmology, University of Ilorin
Associate Editors:
Jeff Pettey, MD, University of Utah Department of Ophthalmology and Visual Sciences, John Moran Eye Center's Residency Program Director
Grace Sun, MD, Weill Cornell Eye - Lower Manhattan, Weill Cornell Medical College Residency Program Director
Assistant Editors:
Samir Patel, BS, Weill Cornell Medical College, New York, New York
Peter Coombs, MD, Weill Cornell Medical College; New York, New York
Regional Contributors:
Sub-Saharan Africa:
Dr. Oluleye Tunji S., University of Ibadan and University College Hospital, Ibadan, Nigeria
American Academy of Ophthalmology
P.O. Box 7424
San Francisco, CA 94120-7424
415.561.8500
Copyright © 2016 American Academy of Ophthalmology®. All Rights Reserved.