Download PDF
Once again, it’s looking like anti-VEGF injection therapy can preserve the vision of patients with a retinal disease—and this time, the good news is about proliferative diabetic retinopathy (PDR).
Noninferiority proved. The positive results came from the 2-year outcomes of a large randomized multicenter trial comparing the long-standard treatment, panretinal photocoagulation (PRP), against intravitreous (IVT) injections with ranibizumab (Lucentis). The study in 394 eyes with PDR found that IVT anti-VEGF therapy was as effective as laser treatment and that there were fewer side effects in the IVT group.1 It’s important to note that the trial was designed to assess the noninferiority of visual outcomes with ranibizumab versus PRP.
Mean improvement in visual acuity at 2 years was no worse in the ranibizumab group (+2.8 letters) than the PRP group. The study also found that patients in the ranibizumab group had less visual field loss, had a lower rate of vitrectomy (4% vs. 15%), and were less likely to develop diabetic macular edema (DME; 9% vs. 28%) compared with the PRP group.
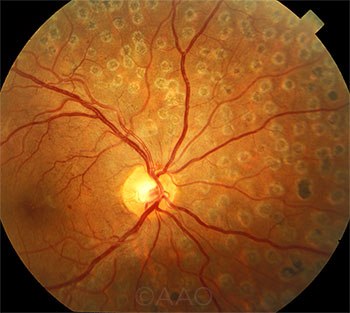 |
PRP. The primacy of laser therapy for PDR may be challenged by recent findings.
|
Expanded treatment options for PDR. When researchers from the Diabetic Retinopathy Clinical Research Network (DRCR.net) announced their findings at AAO 2015 in Las Vegas, the reaction was swift and enthusiastic.
“I think that was because this is the first really major advance offering a treatment alternative to PRP for proliferative diabetic retinopathy in about 40 years,” said Jeffrey G. Gross, MD, who chaired the trial for DRCR.net. “It gives physicians another option, another tool in their toolbox, to treat proliferative diabetic retinopathy.” Dr. Gross is the founder and managing partner at Carolina Retina Center, in Columbia, S.C.
“Our study also showed that over the course of 2 years—not just at 2 years—injection therapy was more effective than panretinal photocoagulation for vision,” he said, adding that patients will be followed for a total of 5 years to assess long-term results.
Choosing between PRP and ranibizumab. Because IVT ranibizumab already is approved for treating DME, clinicians who want to apply these study results to their practice might consider the presence or absence of DME in deciding between ranibizumab and laser therapy.
The study stated: “When DME is present for which ranibizumab treatment is planned, PRP may be unnecessary because ranibizumab, already being given for the treatment of DME, will also treat the PDR.”
The likelihood of patient compliance with all necessary follow-up visits also is an important factor to consider, Dr. Gross said. “This was a structured protocol of injections, which mandated a structured schedule of follow-up,” Dr. Gross said. “In clinical usage it should also be effective, but it’s important that the patient can adhere to this type of structure.” He added that if patients are not able to adhere to a regular follow-up regimen, “it’s unknown whether the same results will be obtained.”
—Linda Roach
___________________________
1 Writing Committee for the Diabetic Retinopathy Clinical Research Network. JAMA. 2015;314(20):2137-2146.
___________________________
Relevant financial disclosures—Dr. Gross: Regeneron: S.
For full disclosures and disclosure key, see below.
More from this month’s News in Review