Download PDF
When doctors in Miami observed high rates of neovascular glaucoma (NVG) in patients who had experienced a central retinal vein occlusion (CRVO), they set out to identify risk factors that could predict the blinding complication. Three risk factors were associated with that progression—and affected patients should be followed at closer intervals and informed of the greater risk of neovascularization, the researchers said.
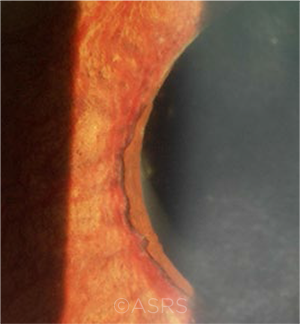 |
ADVANCED CASE. Iris ectropion and prominent iris neovascularization are evident in this case of NVG. This image was originally published in the ASRS Retina Image Bank. Yusuke Oshima, MD, PhD, and Yusuke Takada. Advanced State of Neovascular Glaucoma. Retina Image Bank. 2013; Image Number 5243. © The American Society of Retina Specialists.
|
Risk of progression. In a five-year retrospective review of medical records, the researchers found that 13 of the 98 CRVO patients in their series (13%) progressed to NVG.1 The mean adjusted time from CRVO-related symptoms to diagnosis of NVG was 212 days.
Three key risk factors emerged.
- History of systemic hypertension. This factor has not previously been reported.
- Relative afferent pupillary defect (RAPD). Patients with a RAPD had a relative risk increase of 2.15, at least doubling the probability that the eye will develop NVG. The researchers suggested that a simple pupil exam at each visit should identify the presence of RAPD and determine the course of follow-up care.
- Poorer visual acuity. For every 0.5 logMAR visual acuity worse on presentation, the risk of NVG increased 1.7 times.
No association. Age, body mass index, history of diabetes, and degree of diabetic retinopathy were not associated with NVG. In addition, history of glaucoma did not significantly differ among patients who did and did not develop NVG.
Note on macular edema. Of the 98 CRVO patients, 67 (68%) had macular edema (ME) on initial presentation. Of these, 54 were imaged with optical coherence tomography. When these 54 patients were subdivided according to their NVG status, mean central retinal thickness was 632 ± 221 μm in patients with NVG and 632 ± 335 μm in those without NVG. This corroborates earlier findings that ME and NVG are independent, unrelated sequelae of CRVOs. “Thus, the clinician should not be lulled into a false sense of security after resolution of macular edema,” as improved ME is not a surrogate for decreased neovascular risk, said Andrew J. Rong, MD, at Bascom Palmer Eye Institute in Miami.
Note on anti-VEGF treatment. As anti-VEGF therapy is used to treat CRVO-related ME, the researchers hypothesized that an anti-VEGF injection given on presentation could “decrease the acute ischemic burden in CRVO and provide a long-lasting protective effect against NVG development,” said Dr. Rong. “Instead, we saw that anti-VEGF therapy merely delayed the onset of NVG.” Despite this finding, the researchers advised injecting patients when following CRVO patients.
—Miriam Karmel
___________________________
1 Rong AJ et al. Am J Ophthalmol. Published online March 9, 2019.
___________________________
Relevant financial disclosures—Dr. Rong: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Esmaeli None.
Dr. Ferdi Northcote Trust: S.
Dr. Rong None.
Dr. Tam None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review