By Linda Roach, Contributing Writer, interviewing David H. Abramson, MD, Zélia M. Corrêa, MD, PhD, Dan S. Gombos, MD, and Eric C. Peterson, Md, MS
Download PDF
Over the past decade, 2 bold new techniques for treating retinoblastoma—one still controversial, the other previously eschewed as taboo—have in many centers largely supplanted enucleation and systemic chemotherapy as primary therapy for advanced intraocular retinoblastoma.
Prospective clinical trials of these globe-sparing approaches are lacking. Nonetheless, physicians who have adopted the 2 modalities—intra-arterial chemotherapy (IAC) and intravitreal chemotherapy (IVitC)—as their preferred treatments report overwhelmingly positive outcomes.
Significant Shift
Together and separately, the 2 procedures can spare most children from the disfigurement and harsh side effects of enucleation and systemic chemotherapy while still eradicating their cancer, these specialists say.
“We’ve had a complete reversal from 10 years ago. It used to be that 95% of eyes with unilateral retinoblastoma were removed, now it’s 5%. And this has been done without compromising patient survival. Fewer than 1% of the children worldwide who have had these treatments have died of retinoblastoma,” said David H. Abramson, MD, at Memorial Sloan Kettering Cancer Center in New York City. His retinoblastoma program has performed IAC more than 1,800 times since 2006.
Zélia M. Corrêa, MD, PhD, who leads a retinoblastoma treatment team at the University of Cincinnati, agreed. “With the combination of the intra-arterial and intravitreal treatments, we are able to be much more effective in salvaging eyes in children with bilateral and even advanced unilateral disease, who previously would have been considered only for enucleation.” That said, the following caveat must be heeded, she noted: It’s essential to find the balance between the “risks and benefits of conservative treatment, systemic side effects, and quality of life for the child.”
At the 2017 meeting of the International Society of Ocular Oncology, held in March, it appeared that opinion has begun to shift toward IAC, said Dan S. Gombos, MD, at M.D. Anderson Cancer Center in Houston. “My impression of that meeting was that there was an increasing consensus, a trend, toward using IAC for advanced unilateral disease as first-line therapy,” he said.
However, even though many clinicians employ IAC as their primary therapy, Dr. Gombos pointed out that others “simply will not do IAC.” Still others “employ intravenous chemotherapy primarily but will use IAC in selected cases,” he said.
 |
|
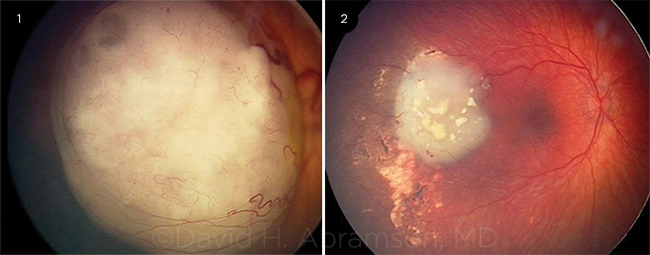
IAC RESULTS. (1) This image shows a large retinoblastoma obscuring the macula. (2) Three months after treatment, the macula is visible, and there is no ocular damage.
|
IAC Basics
Retinoblastoma treatment paradigms began moving away from enucleation and systemic chemotherapy 11 years ago, after Dr. Abramson expanded on previous work by Japanese researchers1 to develop his IAC treatment protocol.2
Technique. With fluoroscopic guidance and the child under general anesthesia, an interventional neuroradiologist (in some centers a neurosurgeon) threads a tiny catheter through the femoral artery, past the heart to the internal carotid artery, and finally up to the orifice of the ophthalmic artery. Then, for 20 to 30 minutes, small doses of the therapeutic drug are pulsed slowly into the ophthalmic artery, for circulation through the orbit into the eye.
“This technique streamlined drug delivery to these eyes. Even though the dose given [with IAC] is about 10% of the dose of intravenous [IV] chemotherapy, the concentration inside the eye is substantially higher, with minimal dispersion into the body, thus minimizing the side effects seen with systemic chemotherapy,” Dr. Corrêa said.
Drug choice. The initial drug Dr. Abramson chose was the alkylating agent melphalan. Today, topotecan and/or carboplatin also can be added.3
A Note on Nomenclature
As treatment protocols develop and mature, so does the terminology used to describe them. IAC is also known as ophthalmic artery chemosurgery (OAC), superselective OAC (SS-OAC), and selective ophthalmic artery infusion chemotherapy (SOAIC).
|
Adding Injections
“You never can assume a child with retinoblastoma is cured until you get rid of all the vitreous seeds,” Dr. Corrêa said. And before IAC, very few eyes with diffuse vitreous seeding—from which new tumors could grow—could be saved. “Some say fewer than 10%,” Dr. Abramson said. “IAC bumped that percentage up to over 60% and then 80%.”
Until relatively recently, attempting to prevent seeding by intravitreal injection was considered too risky, because of the danger of releasing cancer cells that could cause orbital spread of the tumor.
Enter IVitC. The solution came in 2010, when a group led by Francis L. Munier, MD, developed a 3-step technique that involved 1) reducing intraocular pressure before the injection, 2) using a very thin needle, and 3) applying cryotherapy to seal the hole around the needle as it was removed. They also used small volumes of drug (< 0.07 cc) and high-frequency ultrasound to identify safe injection sites.4
“When you withdraw the needle, there’s no hole in the eye for the tumor to come out. It’s a frozen bridge,” Dr. Abramson said. “Everyone picked up his technique, and there have been more than 2,000 intravitreal injections done since 2010. At this point in time, no one has reported growth outside the eye from one of these needle injections.”
Rapid acceptance. IVitC has moved rapidly into the mainstream of retinoblastoma therapy, and it is used in conjunction with both IAC and systemic chemotherapy, Dr. Gombos said.
“I personally think that intravitreal chemotherapy has been a far more transformational therapy than IAC,” Dr. Gombos said. “Because historically when you look at the eyes that failed the old gold standard—IV chemotherapy—they failed primarily because of vitreous disease. And now we have a modality that works really well.”
Starting a Globe-Saving Program
Since 2008, more than 250 IAC procedures have been performed to treat retinoblastoma at the Cincinnati Children’s Hospital Medical Center, said Dr. Corrêa. A well-coordinated multispecialty team is crucial when caring for infants with the disease, some of whom are as young as 1 month old, she said. “When we were just doing external beam radiation or removing the eye, we weren’t as sophisticated. We didn’t need a care team because it was all centered around the ophthalmologist,” she said. “Now that we’re trying to save these eyes, it is extremely important to have a coordinated multispecialty team.”
Reality check. At minimum, such a team should include the following in addition to the ophthalmologist/ocular oncologist: a pediatric oncologist to calculate doses and oversee systemic monitoring; an experienced interventional neuroradiologist or neurosurgeon to plan and perform the arterial catheterizations; and coordinators and social workers to help families with scheduling, transportation, and lodging needs, she said.
“You can’t set up a program like this overnight. Nor can you afford to underestimate the complexity of these treatments and the potential for unintended side effects, or the preparation you need before doing it,” Dr. Corrêa said.
|
Challenges Remain
The benefits of this revolution have not come without costs along the way, because of the complex decision-making and high levels of technical difficulty inherent in these treatments.
Learning curve. The literature on IAC is peppered with case reports of transient and, in a few cases, lasting complications, but experienced clinicians attribute them to physician inexperience.
In one typical case series, as many as 5% of patients experienced transient complications such as eyelid edema, and up to 2% had more lasting complications such as vitreous hemorrhage and partial choroidal ischemia. Later, that group’s total complications fell to a combined total of 1%.5
“Doing this [procedure] is like skiing. Your first couple of times you’re not so slick,” Dr. Abramson said. “The complication rate is directly related to how many you do.”
In discussing complications, Dr. Abramson also invoked the larger context of being able to avoid the impact of systemic chemotherapy and/or enucleation. “Loss of vision does occur in 1% of eyes [with IAC], but all of the other 99% would have been removed” before the treatment was available, he noted.
Technical challenges. For IAC, these include the following.
Navigating small blood vessels. Eric C. Peterson, MD, MS, who has performed about 500 IAC procedures at Bascom Palmer Eye Institute in Miami, said that threading the infusion catheter through tiny arteries is the first technical hurdle. The surgeon must be very careful not to damage the infants’ arteries or cause them to spasm, he said. “We are seeing smaller and smaller babies at earlier stages of the disease, some of them younger than 3 months old. At some point, the diameter of the femoral artery, which should be at least 3 mm, becomes the limiting factor.”
In a few cases, fluoroscopy reveals that the ophthalmic artery, which branches from the internal carotid, is too small for catheterization, he said. “But it turns out that there is a second arterial system, off the external carotid, that also supplies the orbit. So when I see that the kid doesn’t have a great ophthalmic artery, I inject dye into the external carotid artery system to find a target for the catheter. I then can infuse the chemo through that little branch.”
Infusion reflux. Rarely, after the catheter reaches its target, fluoroscopy shows that the toxic infusion might reflux into the internal carotid artery and travel to the brain, Dr. Peterson said. In such cases, he and others who perform IAC inflate a tiny balloon briefly after each drug pulse, then quickly deflate it to allow normal blood flow.
Wrong blood vessel. A recent case referred to Dr. Corrêa showed what can go wrong when the catheter is not in an optimal position, she said. “During the intra-arterial infusion, apparently the drug was not delivered to the eye, either because of catheter misplacement or because of individual variation of this child’s vascular architecture, and a significant dose went to the child’s forehead instead.” Sequelae included necrosis of the skin and frontalis muscle, and the lack of treatment of the eye allowed tumor progression. “After several months, this child now has a prominent keloid on the forehead, and we are still fighting to control the tumors in that eye,” she said.
Immediate side effects. Many clinicians remain troubled by the potential side effects of globe-sparing treatments, including the following.
Neutropenia. This life-threatening adverse effect is of particular concern. Dr. Gombos noted that Dr. Abramson’s group has reported a 29% rate of severe (Grade 3 or 4) neutropenia, an indicator that the chemotherapy drug entered the systemic circulation. However, Dr. Abramson reported, “The blood counts drop to acceptable levels in 99% of patients. That is, only < 1% of patients will need transfusions or fever/neutropenia treatment.”
Injection toxicity. After reports of localized damage after IVitC melphalan, oncologists developed ways to prevent pockets of the drug from pooling against the retina and causing localized toxic side effects. “Because kids have very thick vitreous, we have to grasp the eye with the forceps and then shake the eye gently, to allow the chemotherapy agent we just injected to mix with vitreous,” Dr. Corrêa said.
Research also has shown that melphalan toxicity is a reason to limit the number of injections that children receive to 3, Dr. Abramson said. Each injection results in approximately 5-μV degradation in retinal response.6
Future outcomes. What about metastatic risk? When leaders of 4 of the largest treatment centers in the world compiled data on their first 634 children treated with IAC,3 they found only 1 child who died from a metastasis. In that Argentina case, the child had initially been treated unsuccessfully with systemic chemotherapy, and the parents would not permit enucleation when subsequent IAC failed.
Nonetheless, critics of IAC remain doubtful about the wisdom of salvaging eyes that they believe will have little or no visual potential after treatment, Dr. Gombos said. “When we don’t remove those eyes, or we don’t treat those eyes with systemic chemotherapy, the concern of some people is that we’re putting those children at risk for spread—and we’re putting them at risk for death,” he said. “I wish we had a better picture of all that. But we have to acknowledge that people in the field have this concern.”
Dr. Corrêa agreed. As with many newly developed treatments, unintended consequences emerge over time, she said. “Recently, we had a very unexpected outcome: During IAC and IVitC, a child developed systemic spread of the disease, due to the proximity of the vitreous seeds to the optic disc. When that happened, the tumor became very aggressive” and rapidly metastasized. Since then, Dr. Corrêa said, “We have changed our protocol so that we are even more vigilant of the optic nerve.”
Reasons for hope. Overall, Dr. Abramson remains upbeat about the visual outcomes now and in the future. “Most amazing to me is that in eyes with total retinal detachment, no vision, afferent pupillary defects, and extinguished electroretinograms—which is the classic definition of a blind, hopeless eye—we have published that about 25% of these eyes regain some level of sight. This means that eyes that we’ve always thought were hopeless now are not so hopeless.”
___________________________
1 Kaneko A, Suzuki S. Jpn J Clin Oncol. 2003;33(12):601-607.
2 Abramson DH et al. Ophthalmology. 2008;115(8):1398-1404.
3 Abramson DH et al. JAMA Ophthalmol. 2015;133(11):1341-1347.
4 Munier FL et al. Br J Ophthalmol. 2012;96(8):1084-1087.
5 Shields CL et al. Ophthalmology. 2014;121(7):1453-1460.
6 Francis JH et al. Ophthalmology. 2017;124(4):488-495.
___________________________
Dr. Abramson is chief of the ophthalmic oncology service at Memorial Sloan-Kettering Cancer Center and professor of ophthalmology at Weill Cornell Medical Center in New York, N.Y. Relevant financial disclosures: None.
Dr. Corrêa is a professor of ophthalmology and director of ocular oncology at the University of Cincinnati College of Medicine in Cincinnati, Ohio; and a consulting physician for Cincinnati Children’s Hospital Medical Center. Relevant financial disclosures: None.
Dr. Gombos is professor and chief of ophthalmology at M.D. Anderson Cancer Center in Houston; clinical codirector of the Retinoblastoma Center of Houston; vice chair of a Children’s Oncology Group trial of IAC; and president-elect of the American Association of Ophthalmic Oncology and Pathology. Relevant financial disclosures: Children’s Oncology Group: S; Houseman/Wilkins Ophthalmological Foundation: S.
Dr. Peterson is assistant professor of clinical neurological surgery and director of endovascular neurosurgery at the University of Miami. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.