By Timothy M. Boyce and Alex W. Cohen, MD, PhD
Edited by Steven J. Gedde, MD
Download PDF
When Mary Blaire* first consulted her optometrist, she complained of blurry vision and a sensation of pressure in both eyes and reported that the symptoms had bothered her for one to two months. The optometrist noted that the 49-year-old’s corneas were “swollen” and placed her on 5 percent sodium chloride ointment and artificial tears. After six weeks of this treatment, there was no appreciable improvement, so Ms. Blaire was referred to our service.
We Get a Look
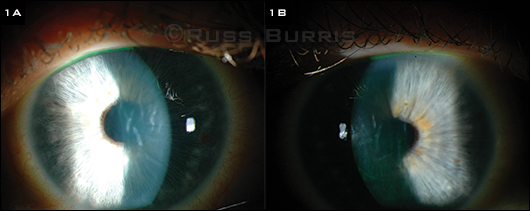
When we saw Ms. Blaire, her best-corrected visual acuity (BCVA) was 20/250 in the right eye and 20/200 in the left. There were no relative afferent pupillary defects, and the intraocular pressure (IOP) was 11 mmHg in the right eye and 9 mmHg in the left. The slit-lamp examination revealed bilateral corneal edema with 3+ Descemet folds without the presence of guttae or other endothelial changes (Figs. 1A and 1B). The rest of the anterior and posterior segment exam was unremarkable.
Ms. Blaire had obvious choreiform movements, and her medical history was extensive. In 2008, she told us, she had a stroke that led to a hyperkinetic movement disorder, and she was taking medication to control her symptoms. She also had a history of anxiety, arthritis, asthma, chronic obstructive pulmonary disease, depression, hypertension, migraines, and seizures. As a result, she was taking a long list of medications, including acetaminophen/butalbital, acetaminophen/hydrocodone, albuterol, amantadine, amlodipine, aspirin, citalopram, clonazepam, cyclobenzaprine, docusate, fluticasone/salmeterol, hydrochlorothiazide, lisinopril, montelukast, omeprazole, rosuvastatin, tiotropium bromide, and topiramate.
|
What's Your Diagnosis?
|
 |
|
PRESENTATION. (1A, 1B) The patient’s right and left eyes, with stromal edema and folds in Descemet membrane.
|
Differential Diagnosis
Given the findings of our exam, we considered the following entities:
Fuchs endothelial dystrophy. This is a common cause of bilateral corneal edema. It is an autosomal dominant disease that is marked by the progressive loss of corneal endothelial cells and the development of guttae. Early Fuchs dystrophy may be asymptomatic, but with endothelial decompensation it can present with decreased vision and pain from bilateral corneal edema. Examination will reveal guttae and folds or wrinkles in Descemet membrane. In later stages of the disease, subepithelial fibrosis and painful bullae may occur. Patients will notice a progressive decrease in vision that does not correct with refraction. Fuchs dystrophy is a common indication for corneal transplantation.
Congenital hereditary endothelial dystrophy. CHED is a rare inherited corneal dystrophy that presents with bilateral corneal opacification at birth or in the first decade of life.
Iridocorneal endothelial syndrome. ICE syndrome is a unilateral disease in which the corneal endothelium takes on the histopathologic features of corneal epithelium. The disease, which progresses slowly, typically presents in middle-aged women and may be associated with prior infection with herpes simplex virus (HSV) or Epstein-Barr virus. Over time, the cornea may begin to become edematous.
HSV endotheliitis. This is thought to be an immune-mediated reaction to viral antigen deposited in or around the corneal endothelial cells during a prior infection. Patients can present with three forms of endotheliitis: disciform, diffuse, or linear. Disciform endotheliitis generally has a round distribution of keratic precipitates, microcystic edema, and stromal edema. Diffuse endotheliitis presents with diffuse keratic precipitates, microcystic edema, and stromal edema. Linear endotheliitis presents with a line of keratic precipitates that originates close to the limbus and advances centrally. Between the keratic precipitates and the limbus, stromal and microcystic edema develops. In addition, patients may experience pain, photophobia, injection, and decreased vision. HSV-related endothelial disease is usually a unilateral condition.
 |
|
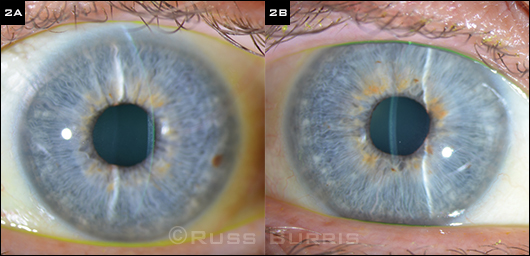
Problem Solved. (2A, 2B) These slit-lamp images, taken at the last follow-up visit, show resolution of the edema in each eye.
|
Dead Ends and a New Suspect
These initial suspects were ruled out. To begin with, Ms. Blaire’s corneas did not have guttae; as a result, we ruled out Fuchs dystrophy.
With regard to CHED, our patient’s age (49) made this diagnosis improbable. We also felt that ICE syndrome was unlikely, given that Ms. Blaire had bilateral disease and normal-looking endothelium.
Finally, with regard to HSV endotheliitis, Ms. Blaire had bilateral symmetric corneal edema without pain or photophobia. In addition, she did not have a history of ocular or oral-labial herpes.
At this point, we turned our attention to Ms. Blaire’s long list of medications. Several substances have been found to cause toxic injury to the corneal endothelium, resulting in edema. Amantadine, which is used to treat movement disorders, has been reported to cause corneal edema in some patients, and we considered this the likeliest suspect. (Other medications that cause corneal edema are chlorhexidine and benzalkonium chloride; the latter is a common preservative in eyedrops.) Ms. Blaire had started treatment with amantadine about three years before the onset of her blurred vision.
Follow-up and Treatment
We diagnosed Ms. Blaire with amantadine-induced corneal edema and asked that she discuss discontinuation of this medication with her neurologist. At that time, the neurologist felt that the drug dose could be cut in half; however, he was reluctant to discontinue the medication altogether, in part because he didn’t believe that it was the culprit in her visual decline.
Worsening symptoms. Four weeks later, Ms. Blaire returned to our service. Both her movement disorder and corneal edema had worsened, as noted both symptomatically and by the slit-lamp exam. At this point, her BCVA was 20/250 in the right eye and 20/200 in the left, and her central corneal thickness (CCT) measured 765 µm in the right eye and 831 µm in the left. Given the worsening of both the ocular and neurologic disease, we directly discussed the toxicity of this medication with her treating neurologist and asked if she could be transitioned to another medication.
Six weeks later, Ms. Blaire returned, noting a further decrease of her vision as well as increased redness and irritation. Her vision was now 20/250 in each eye, and her CCT was 771 µm in the right eye and 910 µm in the left. Ms. Blaire told us that she had been placed back on full-dose amantadine because of the worsening of her movement disorder.
Switch in medication. After another discussion with her neurologist, the decision was made to switch medications in hopes of avoiding a bilateral corneal transplantation. Ms. Blaire was taken off of the amantadine and placed on haloperidol.
Problem solved. Ms. Blaire returned to our clinic approximately three months later. At that visit, we noted nearly complete resolution of the edema, and her BCVA had improved to 20/25 in the right eye and 20/30 in the left. The CCT was now 591 µm in the right eye and 593 µm in the left. Ms. Blaire’s movement disorder was well controlled on her new medication, and she was very happy to have avoided surgery.
Discussion
Amantadine was initially used for the prophylaxis and treatment of influenza A but is rarely prescribed for influenza today, as more than 90 percent of strains are now resistant. Currently, it is used to relieve the symptoms of Parkinson disease, dyskinesias, and choreiform movements. The mechanism of action is not completely understood, but the drug is believed to potentiate the release of dopamine and antagonize N-methyl-D-aspartate receptors.1-3
In the past 20 years, various case reports have documented endothelial toxicity due to amantadine. In 2007, the Veterans Health Administration conducted a large retrospective study of 13,137 patients who were given the drug. The researchers found that 0.27 percent of the patients were diagnosed with Fuchs corneal dystrophy or corneal edema, and the relative risk of corneal edema from amantadine use was found to be 1.7.4
The onset of corneal edema after beginning amantadine varies widely, ranging from as little as two weeks5,6 to a period of many years.7,8
The mechanism of amantadine toxicity on corneal endothelium remains unknown. It has been postulated that the drug may cause a delayed hypersensitivity reaction. In 2010, a cross-sectional study measured CCT, endothelial cell density, coefficient of variation, and hexagonality in 169 patients who were taking different doses of amantadine for Parkinson disease. Compared to the control group, the amantadine group had significantly lower endothelial cell densities, lower hexagonality, and a greater coefficient of variation, and these results were found to be dose dependent.9 The researchers concluded that amantadine may lead to endothelial toxicity by two mechanisms: 1) a drug hypersensitivity reaction, which causes corneal edema in a short period of time, and 2) a dose-dependent loss of endothelial cells, which takes a period of years.9
With regard to treating amantadine-induced endothelial toxicity, the only definitive strategy is to discontinue use of the drug and hope for recovery of endothelial cell function. Some patients may have irreversible corneal injury and must undergo a corneal transplant.10 Fortunately, in others (as in our patient), the corneal edema reverses, and surgery is avoided.
___________________________
* Patient’s name is fictitious.
___________________________
1 Engber TM et al. Neuroreport. 1994;5(18):2586-2588.
2 Blanpied TA et al. J Neurosci. 2005;25(13):3312-3322.
3 Grelak RP et al. Science. 1970;169(3941):203-204.
4 French DD, Margo CE. Cornea. 2007;26(9):1087-1089.
5 Blanchard DL. Cornea. 1990;9(2):181.
6 Fraunfelder FT, Meyer SM. Am J Ophthalmol. 1990;110(1):96-97.
7 Chang KC et al. Cornea. 2008;27(10):1182-1185.
8 Dubow JS et al. Mov Disord. 2008;23(14):2096-2097.
9 Chang KC et al. Ophthalmology. 2010;117(6):1214-1219.
10 Jeng BH et al. Ophthalmology. 2008;115(9):1540-1544.
___________________________
Mr. Boyce is a medical student at the University of Oklahoma, and Dr. Cohen is a specialist in cornea and external diseases at the Dean McGee Eye Institute and the University of Oklahoma in Oklahoma City. The authors report no related financial interests.
Calling All Cases!
Interested in sharing a case with your colleagues? Submit it at:
Morning Rounds EyeNet Magazine655 Beach Street San Francisco, CA 94109 Phone: 866-561-8558 or 415-561-8500 eyenet@aao.org |