By Annie Stuart, Contributing Writer, interviewing Bala Ambati, MD, PhD, Sarwat Salim, MD, FACS, and Prithvi S. Sankar, MD
Download PDF
Now is a good time to examine the interplay between myopia, refractive surgery, and glaucoma. Why? To begin with, “Myopia is a well-known risk factor for glaucoma and is currently becoming an epidemic,” said Sarwat Salim, MD, FACS, at the New England Eye Center in Boston. Second, more than 220,000 LASIK, SMILE, and PRK procedures were per-formed in the first quarter of this year—a nearly 30% increase since last year.1
“As more myopic individuals have refractive surgery, it’s important for surgeons to not only screen them for glaucoma but also to monitor them regularly over time to reduce their risk of developing glaucoma,” Dr. Salim said.
 |
|
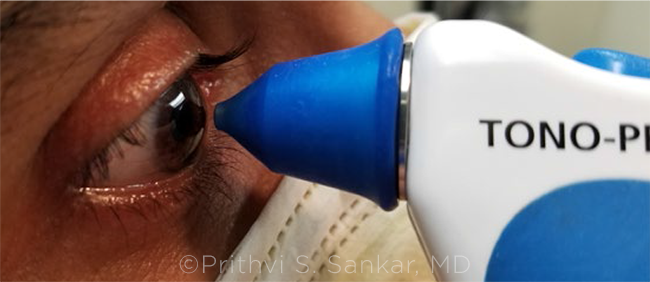
IN PURSUIT OF ACCURACY. After refractive surgery, measure IOP with more than one instrument. The Tono-Pen may provide a more accurate reading than Goldmann applanation tonometry.
|
Screening and Testing
Prithvi S. Sankar, MD, at the Scheie Eye Institute in Philadelphia, recommends a thorough history, several baseline glaucoma tests, and ongoing monitoring for myopic patients who undergo refractive surgery.
“This may seem daunting for both patients and physicians,” he said. However, he said, he has found that patients appreciate having access to test results “to help them make more informed decisions about these elective surgeries.” He added that baseline testing is key not only for deciding about surgery but also for making comparisons in the future.
Medical and family history. If a patient is a glaucoma suspect, has a family history of glaucoma, or has diabetes or hypertension, Bala Ambati, MD, PhD, who practices in Eugene, Oregon, advises them that they are at increased risk for developing glaucoma in the future. It’s particularly important to emphasize that the presence of myopia increases this risk, Dr. Salim said. “I strongly advise that myopic patients have baseline testing for glaucoma and close monitoring.”
OCT. Getting baseline OCT images allows clinicians to observe changes longitudinally, even in the presence of artifacts induced by myopia, said Dr. Salim. “Keep in mind that eyes with moderate to high myopia may not be well represented in the normative reference database,” she said. (See “How to Spot Glaucoma in the Myopic Patient,” May EyeNet.) She added that it is also important to avoid categorizing individuals as having glaucoma when they do not have the disease. The retinal nerve fiber layer thinning may be due to myopia.
“In myopes, the nerve fiber layer bundle tends to be more concentrated in certain areas, such as the perimacular region,” Dr. Sankar noted. “Because it can look abnormal initially on OCT, documentation can be instrumental for spotting subtle changes over time, even in those you don’t suspect as having glaucoma. But without the pre-op baseline, you won’t have that point of comparison.”
Perimetry. Because OCT detects structural changes and visual fields disclose functional loss, getting both tests helps provide corroboration, Dr. Sankar said. He added that it’s important to get multiple visual field tests. “Changes on visual fields may also occur quite quickly after LASIK and be long-lasting. Subtle changes may not be indicative of glaucoma in these individuals; instead, they may be a ‘new normal.’”
Clinicians need to be aware that many of the visual field defects that can occur with myopia alone are similar to those observed in glaucoma. These may include a large blind spot, nasal step, arcuate defect, or paracentral defect, said Dr. Salim.
Gonioscopy. “Although glaucoma specialists routinely use gonioscopy to check whether the eye’s drainage angle is open or closed, refractive surgeons typically don’t,” said Dr. Salim. “Because hyperopes have smaller eyes, narrower angles, and a more congested anterior segment, cases of acute angle-closure glaucoma after LASIK have been reported.”2,3 A small cadre of myopes also have narrow angles, said Dr. Sankar. Therefore, gonioscopy is critical pre- and postoperatively.
Gonioscopy also is helpful for assessing the degree of pigmentation and for diagnosing pigment dispersion syndrome (PDS), especially if other clinical signs of PDS are not visible on a slit-lamp exam, said Dr. Salim. PDS is common in myopes and increases their risk for pigmentary glaucoma and steroid-induced glaucoma, she said.
Disc photography. With the advent of OCT, disc photography is done less frequently, but it is still an outstanding technique, Dr. Sankar said. “OCT and other technologies may evolve over time, but disc photos provide a very nice snapshot, allowing us to know exactly what a patient’s optic nerves looked like at a certain point in time, and they are helpful for future comparisons. Having a good set of baseline disc photos to compare with later can be priceless.”
These photos are especially invaluable for myopes, whose optic nerves may be difficult to interpret clinically, said Dr. Salim. “They allow us to objectively follow eyes much better than written descriptions, which can differ due to interobserver variability.”
Tonometry. It’s important to get a series of pre- and postsurgical measurements for all refractive surgery candidates. This is especially true for those at increased risk of developing glaucoma, said Dr. Sankar. Remember that refractive surgery can change the architecture and thickness of the cornea, he added, and that this change can cause post-op IOP to be underestimated, particularly when testing is done with Goldmann applanation tonometry (GAT).
“Still the gold standard, GAT was designed to be most accurate when measuring a cornea with a central corneal thickness of 520 μm,” said Dr. Salim. “After refractive surgery, it’s helpful to measure IOP with more than one instrument—ones that are less likely to be affected by stromal ablation.” Potential options include the following:
Tono-Pen. This handheld, portable applanation tonometer can potentially give a more accurate reading after LASIK, said Dr. Sankar. However, placing the Tono-Pen (Reichert) on the limbus beyond the flap will produce high pressure readings, Dr. Ambati noted.
Dynamic contour tonometry. Because the Dynamic Contour Tonometer (Ziemer) does not involve applanating corneal tissue, its measurement is independent of corneal properties, which makes it a good choice after refractive surgery, said Dr. Salim.
Ocular response analysis. The Ocular Response Analyzer (Reichert) measures corneal hysteresis and IOP, allowing it to account for the cornea’s shock absorbency and making it a good option after refractive surgery, Dr. Salim noted.
IOP: Nuances of Different Procedures
LASIK. With both the microkeratome and the femtosecond laser, IOP elevation may temporarily occur during creation of the LASIK flap, Dr. Salim noted. “The great majority of LASIK flaps today are created with femtosecond laser, which poses a lower risk of IOP elevation [than does the microkeratome]. However, IOP range may vary with different femtosecond laser platforms, and this may put a fragile optic nerve at risk.”
Patients may face another challenge after LASIK, Dr. Ambati said. “Some are at risk for post-LASIK interface fluid syndrome,” which can cause artificially lowered pressure measurements. A loose flap also may have this effect, said Dr. Salim. “The force required to applanate the overlying flap is dampened because of the loose flap or the fluid under the cyst, causing an artificially low pressure reading.”
Post-op care. The patient’s treating ophthalmologist needs to know about the history of LASIK, amount of refractive correction, and the potential for “low” pressure measurements after stromal ablation, said Dr. Ambati. Unfortunately, he noted, many patients “often don’t follow up for routine eye exams, including optic nerve assessment.”
Phakic IOLs. These clear implantable lenses offer an alternative to LASIK and PRK for correcting myopia. The surgeon may place them directly in front of or behind the iris, leaving the natural lens in place. “Although not common, [phakic IOLs] can cause chafing of the iris, leading to PDS and increased eye pressure,” said Dr. Ambati. “If the surgeon improperly places the lens or does not perform preoperative peripheral iridotomy, pupillary block may also occur, prompting a sudden increase in IOP.”
Other mechanisms of glaucoma after placement of phakic IOLs include malignant glaucoma, steroid-induced glaucoma, and pseudophacomorphic glaucoma, added Dr. Salim. Preexisting PDS is a contraindication for placement of phakic IOLs.
PRK. Visual recovery following PRK takes longer, requiring use of steroids postoperatively, Dr. Salim noted. Patients at higher risk for steroid-induced hypertension include those with myopia, glaucoma or a family history of glaucoma, or diabetes, said Dr. Ambati. “In most cases, this usually resolves after stopping the steroids, but some may require treatment with glaucoma medications.”
Refractive lens exchange. Replacing the eye’s natural lens with an IOL “may actually lower the IOP—especially in hyperopes, who have smaller eyes—and may reduce the risk for angle-closure glaucoma,” said Dr. Ambati. “The natural lens may push the iris forward, causing a decrease in outflow that leads to a buildup in pressure. But the IOL is thinner and occupies less space, allowing fluid to more easily exit the eye.”
SMILE. As patients who undergo SMILE (small incision lenticule extraction) are on steroids for only a few days, there is no real issue with steroid-induced glaucoma, Dr. Ambati said. “However, one still has to be aware of lower IOP measurements due to a thinner cornea after tissue removal.”
Given this possibility of artificially lowered eye pressure, SMILE patients need regular follow-up and should share information about their refractive procedure with their ophthalmologists.
|
Top Pearls
Pearls to keep in mind include the following:
If you are a refractive surgeon. Inquire about family history of glaucoma; educate patients about the in-creased risk of glaucoma in the presence of myopia; perform a comprehensive exam, including gonioscopy and IOP measurements using different devices; obtain baseline ancillary tests, including disc photos, OCT imaging, and visual fields; and emphasize the need for regular follow-ups, said Dr. Salim.
Dr. Sankar also recommended encouraging patients to keep records, including IOP readings and any other tests they’ve received.
If you are a glaucoma surgeon. Inquire about myopia and any previous history of refractive surgery, said Dr. Salim. Because refractive surgery patients no longer wear glasses, some forget that they once were nearsighted, or they don’t realize that other structures of their eyes—their optic nerves or angle anatomy, for example—may be affected by this history, Dr. Sankar added.
It’s important to ask specific questions about refractive surgery, Dr. Ambati agreed. “That’s because many patients don’t think of laser surgery as eye surgery. And since a LASIK flap could be invisible, you might not be aware that a laser procedure was done in the past.”
If possible, obtain the patient’s pre- and post-op refractive surgery information, Dr. Salim said. “It may help to know the level of baseline myopia and how much ablation was done to approximate the patient’s real IOP.” Knowing the corneal thickness before and after surgery will help glaucoma specialists interpret the patient’s status, Dr. Sankar agreed.
And Dr. Salim said, in addition to measuring IOP by methods that are least likely to be altered by previous refractive surgery, it’s important to pay more attention to other parameters of glaucoma evaluation.
___________________________
1 https://eyewire.news/articles/refractive-surgery-council-reports-nearly-30-rise-in-laser-vision-correction-procedures-year-over-year. Accessed Aug. 17, 2021.
2 Paciuc M et al. Cataract Refract Surg. 2000;26(4):620-623.
3 Osman EA et al. Saudi J Ophthalmol. 2009;23(3-4):215-217.
___________________________
Dr. Ambati is a research professor at the University of Oregon, president of Pacific Clear Vision Institute, and a refractive surgeon in Eugene, Oregon. Relevant financial disclosures: None.
Dr. Salim is professor of ophthalmology, vice chair of clinical and academic affairs, and director of the glaucoma service at the New England Eye Center, Tufts University School of Medicine, in Boston. Relevant financial disclosures: None.
Dr. Sankar is professor of clinical ophthalmology and a glaucoma specialist at the University of Pennsylvania’s Scheie Eye Institute in Philadelphia. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Ambati Alcon: L; iVeena: O; Peschke: C.
Dr. Salim Aerie: C,L.
Dr. Sankar None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|