Download PDF
The glycemic threshold above which diabetic retinopathy (DR) can be predicted to develop is lower for whites than it is for blacks and Hispanics, researchers have found.
In a retrospective study, a team of researchers at the University of Miami used data from 5,338 participants in the 2005-2008 National Health and Nutrition Examination Survey (NHANES). All had diabetes and had undergone digital retinal imaging to determine their retinopathy status. The analysis showed that the hemoglobin A1c (HbA1c) predictive threshold for the incidence of DR among white participants was 6.0%.1 In contrast, the predictive thresholds for DR in Hispanics and blacks were 6.4% and 6.5%, respectively.
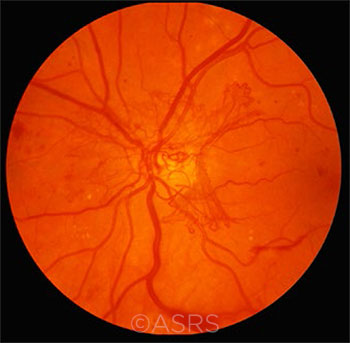 |
GLYCEMIC CONTROL. Tight glycemic control in patients with diabetes is recommended as a way to prevent complications, such as the proliferative DR seen here. But should the thresholds be revisited? This image was originally published in the ASRS Retina Image Bank. Michael P. Kelly, FOPS. Proliferative Diabetic Retinopathy. Retina Image Bank. 2012; Image Number 774. © The American Society of Retina Specialists.
|
Adequate—but inadequate? “Importantly, all three race/ethnicity–specific glycemic thresholds are less than the recommended 7.0% [for optimal HbA1c control] for people with diabetes,” the authors wrote. “This finding suggests that adequate glycemic control does not guarantee protection from diabetic complications, such as DR.”
Indeed, the researchers calculated that above these thresholds the risk of a diabetic patient developing retinopathy was approximately 4 to 6 times as high as it would be below them, said lead author Kevin J. Moore, MD, MPH, now at the University of Central Florida in Orlando.
Advising patients. Dr. Moore said the researchers hope their results will help physicians offer more individualized advice to their patients who have diabetes. “This shows that you can have individuals that are considered well-controlled for diabetes but who, based on their race or ethnicity, are still at risk for diabetic retinopathy,” Dr. Moore said. “We would hope that our paper would inspire ophthalmologists and primary care providers to emphasize to patients that it’s still important to follow up, regardless of how well their diabetes is controlled.”
A look at the guidelines. Racial differences in mean HbA1c levels have been discussed in the diabetes literature, but the reasons for these differences are poorly understood, and their potential clinical significance is unknown.2 The American Diabetes Association (ADA) makes no recommendations that glycemic targets be modified based on race or ethnicity.3
Large, prospective treatment trials have demonstrated that retinopathy and other microvascular complications decrease at HbA1c levels below 7.0%, and this is the appropriate target for most patients, according to the ADA’s 2019 recommended standards of care.3 However, the ADA report acknowledged, the lower complication rates achieved in studies have been accompanied by increases in the incidence of serious hypoglycemia.
Because of this risk, it would be “reasonable” for physicians to recommend a lower glycemic target of 6.5% for certain patients, if it can be met without hypoglycemia or other adverse effects, the ADA report said. This includes patients with a short duration of diabetes, long life expectancy, type 2 disease being treated with lifestyle or metformin only, or no significant cardiovascular disease.
By comparison, the Japan Diabetes Society adopted practice guidelines4 in 2013 that endorse setting the HbA1c target at 6.0% or less when the physician judges that glycemic control can be achieved through diet, exercise, or medication. The guidelines set 7.0% as the ceiling for most other patients, with the goal of preventing complications.
—Linda Roach
___________________________
1 Moore KJ et al. JAMA Ophthalmol. Published online Feb. 21, 2019.
2 Selvin E. Diabetes Care. 2016;39(8):1462-1467.
3 American Diabetes Association. Diabetes Care. 2019;42(Suppl 1):S61-S70.
4 Araki E et al. Diabetol Int. 2016;7(4):327-330.
___________________________
Relevant financial disclosures—Dr. Moore: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Colby WL Gore, Inc.: C.
Dr. Gupta None
Dr. Moore None
Dr. Hesse None
Dr. Rosen Astellas: C; Bayer: C; Boehringer Ingelheim: C; Diopsys: C; Genentech/Roche: C; Guardion Health: O; Nano-Retina: C; OD-OS: C; Opticology: O; Optovue: C; Regeneron: C; Teva: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review