Download PDF
Last month, Part 1 of this two-part series discussed the orientation and acquisition of B-scans, including transverse, longitudinal, axial, and macular scans. This month’s article presents the chief characteristics of ultrasound scans, along with examples of how to use them to recognize and distinguish between pathological conditions.
Characteristics Seen on Ultrasound Scans
Pathology on B-scan can be evaluated by three primary characteristics: topographic, quantitative, and kinetic.
Topography. Topographic characteristics of a lesion include its location in the eye, extension, and shape.
Quantitative features. Quantitative characteristics include reflectivity, internal structure, and sound attenuation. Reflection occurs as a result of density differences at the interface between different tissue structures, with higher reflectivity corresponding to a brighter (or hyperechoic) image on B-scan. On an A-scan, the height of spikes corresponds to reflectivity. The sclera is the most reflective normal ocular structure.
Internal structure of a lesion refers to the reflectivity within the lesion. An irregular internal structure is seen as heterogeneous echogenicity on B-scan or varied spike intensity and height on A-scan. A regular internal structure demonstrates more homogeneous echogenicity on B-scan and more consistent A-scan spike morphology within the lesion.
Sound attenuation is caused by the dispersal of energy through a specific medium. This is seen as decreasing echogenicity within a lesion as well as progressively decreasing A-scan spike intensity through the lesion (with the steepness of spike reduction referred to as the angle kappa). Lesions that cause high sound attenuation (e.g., calcification or metallic foreign bodies) may also produce posterior acoustic shadowing. Although attenuation is helpful in characterizing lesions, high attenuation makes B-scan difficult in eyes filled with silicone oil.
Kinetic characteristics. Finally, kinetic features include after-movement, internal vascularity, and convection motion. After-movement refers to the extent of tissue motion immediately after eye movement. This is particularly useful when differentiating between posterior vitreous detachments, retinal detachments, and choroidal detachments, as detailed in the next section.
Internal vascularity is useful in assessing tumors. With the patient maintaining steady fixation with the fellow eye and the probe held stationary, internal vascularity is seen as fluctuating low-intensity A-scan spikes within a lesion and corresponds to blood flow in that region. Convection motion can be seen as movement within a lesion and typically indicates stagnant blood or cholesterol debris such as in a choroidal effusion or a Coats disease–related serous retinal detachment.
 |
|
PVD. (1) Complete PVD with vitreous hemorrhage. Blood is seen precipitating on the posterior hyaloid face. (2) PVD with hyperreflective posterior adhesion indicating a flap retinal tear (arrow).
|
 |
|
DETACHMENTS AND MASSES. (3) Total RRD seen connecting to the optic nerve. The echogenicity and height of the A-scan spike as well as relatively limited aftermotion on dynamic ultrasonography can help differentiate RRD from PVD. (4A) Choroidal effusion seen extending past the ora serrata anteriorly but limited posteriorly by the exit foramina of the vortex veins. The B-scan image shows the smooth, dome-shaped elevation typical of choroidal detachment. (4B) A-scan passing more perpendicular to the detachment demonstrates a double-peaked M spike. (5) Tractional attachments between the vitreous and retina causing a “tabletop” tractional retinal detachment in a patient with proliferative diabetic retinopathy. (6) Mushroom-shaped choroidal melanoma demonstrating increased internal reflectivity of the herniated portion of the mass compared to the posterior aspect, as well as decreasing A-scan intensity through the lesion (angle kappa). (7) Circumscribed choroidal hemangioma demonstrating high internal reflectivity.
|
 |
|
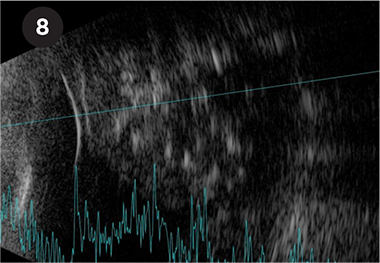
RETINOBLASTOMA. Retinoblastoma demonstrating irregular high internal reflectivity from intralesional calcification.
|
 |
|
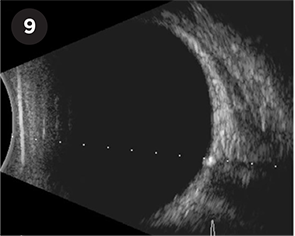
ODD. Optic disc drusen seen as a hyperechoic lesion at the optic nerve head with posterior acoustic shadowing.
|
 |
|
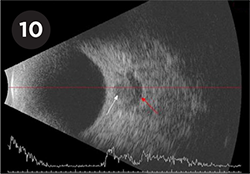
PAPILLEDEMA. Crescent sign in a patient with papilledema seen as a crescent of hypoechoic fluid (red arrow) surrounding the optic nerve (white arrow).
|
Differentiation of Lesions
Detachments. Although posterior vitreous detachments (PVD), rhegmatogenous retinal detachments (RRD), and choroidal detachments (CD) can be challenging to differentiate, certain anatomic and imaging characteristics can be useful (Table 1). From an anatomic perspective, neither PVD nor RRD will extend past the ora serrata, whereas CD can extend more anteriorly. Further, CD can extend posteriorly only to the exit foramina of the vortex veins, but not to the optic nerve. However, RRD can extend posteriorly to attach at the optic nerve; and although an incomplete PVD can attach at the optic nerve, a complete PVD will have no posterior attachments (Fig. 1). Focal posterior adhesions of a PVD should raise concern for areas of retinal tear or traction.
On close examination, flaps of retinal tissue can also be identified in cases of PVDs, indicating the location of a retinal tear (Fig. 2). A PVD will disappear on a low-gain scan, whereas RRD and CD will persist. On dynamic scans, PVD will demonstrate high after-movement, acute RRD will have more restricted after-movement, and CD will be immobile.
An A-scan passing through a PVD will show a much lower amplitude spike compared with an RRD, which would show an amplitude equal to that of the sclera (Fig. 3). In contrast, a CD will demonstrate a wide, double-peaked “M spike” on an A-scan passing perpendicular to the detachment (Fig. 4). In cases of vitreous hemorrhage in which a PVD has already occurred, precipitation of blood on the posterior hyaloid face can increase its reflectivity, mimicking the appearance of a retinal detachment. Nevertheless, the features described above can still be used to correctly identify a PVD in these cases.
Both tractional retinal detachments (TRD) and RRD with proliferative vitreoretinopathy (PVR) show little or no after-movement. TRD will have a peaked configuration, with the apex attaching to the posterior hyaloid (Fig. 5), while cases of PVR can demonstrate retinal thickening and fixed folds.
Intraocular masses. Intraocular masses often represent a diagnostic challenge to ophthalmologists. Ophthalmic ultrasound can provide a noninvasive means of further characterizing these lesions by assessing their morphology, internal structure, and extent. Furthermore, if available, color Doppler imaging can help assess for vascularity within a lesion.
Choroidal nevus vs. melanoma. A choroidal nevus is typically seen as a domelike elevation of uniform medium to high internal reflectivity. In contrast, a choroidal melanoma has a homogeneous structure with low internal reflectivity (Table 2). The lesion thickness of choroidal melanoma may be greater than its diameter (with thickness >2 mm being more suspicious1), and choroidal excavation can also be seen at the base.
If a choroidal melanoma breaks through the Bruch membrane, it can have a “collar-button,” or mushroom-shaped, appearance, with the herniated portion demonstrating increased internal reflectivity due to reduced drainage of blood in front of the membrane. A-scan of choroidal melanoma will demonstrate an angle kappa (Fig. 6). Vascularity can be seen with color Doppler or as flickering reflectivity spikes on A-scan.2
Choroidal metastases. In comparison to choroidal melanomas, choroidal metastases (the most common of which are from breast and lung cancer3) are typically shallow lesions with moderate to high internal reflectivity. They may be associated with a retinal detachment.
Hemangiomas. Choroidal hemangiomas, which can be found in isolation (circumscribed) or in association with Sturge-Weber syndrome (diffuse), are seen as a highly reflective thickening of the choroid with only mild elevation (Fig. 7) and minimal flow on color Doppler.
Retinoblastoma. Retinoblastoma, the most common intraocular malignancy of childhood, demonstrates characteristic irregular high internal reflectivity due to intralesional calcification (Fig. 8).
Optic disc drusen and papilledema. B-scan can be particularly useful in differentiating between optic disc drusen (ODD) and papilledema, conditions that are often confused on direct examination. Calcified ODD will be seen as highly reflective elevated foci at the optic nerve head (Fig. 9). However, it is important to note that younger children often have buried and noncalcified ODD, which are harder to pick up on ultrasound.4
Papilledema will also demonstrate elevation of the optic nerve head but without marked hyperreflectivity. Additionally, a transverse cross section of the optic nerve may demonstrate a crescent or ring of hypoechoic fluid surrounding the nerve (“crescent sign” or “doughnut sign”; Fig. 10). An enlarged optic nerve sheath diameter (>3.3 mm)5 or a positive 30° test (>10%-20% reduction in optic nerve sheath width when the eye is moved from primary gaze to 30° away from primary) have also been shown to be highly sensitive in differentiating papilledema from ODD.6
Conclusion
Ophthalmic ultrasound is an important diagnostic tool for ophthalmologists. Understanding the steps of performing a complete examination is critical to avoid missing pathology that may not be seen on direct examination, and skilled interpretation can be invaluable in diagnosing a variety of chorioretinal lesions and other ocular conditions.
___________________________
1 Shields CL et al. Arch Ophthalmol. 2009;127(8):981-987.
2 Kaliki S, Shields CL. Eye (Lond). 2017;31(2):241-257.
3 Shields CL et al. Ophthalmology. 1997;104(8):1265-1276.
4 Chang MY et al. Ophthalmology. 2017;124(12):1839-1848.
5 Neudorfer M et al. Acta Ophthalmol. 2013;91(4):376-380.
6 Carter SB et al. Eye (Lond). 2014;28(12):1425-1430.
___________________________
Dr. Green is a resident in ophthalmology, Dr. Hussain is a vitreoretinal surgery fellow, and Dr. Ness is Chief of the Retina Service and Director of the Vitreoretinal Surgery and Medical Retina Fellowships; all are members of the Department of Ophthalmology at Boston Medical Center, Boston, Mass. Financial disclosures: None.