Download PDF
In patients with polypoidal choroidal vasculopathy (PCV), a subtype of age-related macular degeneration, combining anti-VEGF injections with photodynamic therapy (PDT) has been found to improve visual outcomes compared to ranibizumab monotherapy at 24 months.1
The EVEREST II study was a follow-up to an earlier trial (EVEREST) that found similar results after following patients for 12 months, said lead author Tock H. Lim, MBBS, FRCSEd, at the National Healthcare Group Eye Institute in Singapore. “The EVEREST studies’ 12-month and now 24-month data have shown that if you treat PCV with combination therapy you get a very good outcome.”
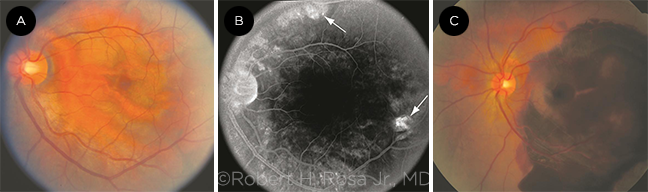 |
PCV. In this patient with polypoidal choroidal vasculopathy, (A) red-orange nodular and tubular lesions are evident in the peripapillary and macular regions. (B) Hyperfluorescent polypoidal lesions (arrows) without apparent leakage on fluorescein angiography. (C) A dense subretinal hemorrhage has occurred.
|
Overview of EVEREST II. Of 322 participants randomized to receive combination therapy or monotherapy in EVEREST II, 274 completed the 24-month study. Those in the monotherapy group (n = 154) received ranibizumab 0.5% intravitreal therapy. Combination therapy patients (n = 168) received intravitreal injections of ranibizumab 0.5% and standard verteporfin PDT.
With regard to the study’s outcomes, Dr. Lim said, “Specifically, if you combine verteporfin PDT with ranibizumab injections you get a 9.6-letter gain,” compared to 5.5 letters in the monotherapy group. Moreover, he said, “You get that visual acuity gain when your baseline is already quite high at 61 letters. And you will use half the median number of injections of ranibizumab that you would use with monotherapy, with no increased risk.”
PCV in East Asia … The EVEREST trials gathered subjects from ophthalmic institutions throughout East Asia, Dr. Lim said. About a third of neovascular AMD patients in East Asian countries have PCV, he said.
“The PCV subtype consists of two lesion components, which are intertwined. In the center, there is often a branching vascular network, similar to the pattern seen in type 1 choroidal neovascularization,” Dr. Lim said. “At the perimeter of the vascular area are aneurysmal dilatations known as polypoidal lesions. Leakage and bleeding can either come from the lesions or from the branching vascular network.”
… and elsewhere. Now that the diagnostic protocol used in the EVEREST trials has been published, researchers in other countries are beginning to find PCV cases in other ethnic groups,2 which might previously have remained unsuspected, Dr. Lim said.
Advice to clinicians. The PCV imaging and diagnostic methods used in the EVEREST trials already are commonly used by ophthalmologists in referral centers in Asia, based on earlier study reports, Dr. Lim said. The protocol is based on confocal scanning laser ophthalmoscopy indocyanine green angiography (ICGA) in tandem with other imaging techniques.
Typically, identification of PCV begins with a fundus examination aided by optical coherence tomography (OCT). “The key is that, if you see round-shaped hyperreflective lesions between the retinal pigment epithelium and Bruch’s membrane in a good set of OCT images, consider ICGA to confirm the diagnosis of PCV,” Dr. Lim said.
He added, “If a person receives anti-VEGF monotherapy and you don’t seem to see the same response that you would expect in a standard case, it’s important to go through the OCT sections and determine whether you see any suspicious polypoidal lesions. And if in doubt, then you should do ICGA or send the patient to a colleague who does this procedure.”
He added, “That’s important, because once you find that the disease is PCV, you now have an additional treatment method that can give the patient a better outcome.”
—Linda Roach
___________________________
1 Lim TH et al. JAMA Ophthalmol. Published online July 16, 2020.
2 Sohraab Yadav et al. Br J Ophthalmol. 2017;101(10):1377-1380.
___________________________
Relevant financial disclosures—Dr. Lim: Heidelberg Engineering: L; Novartis: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Calkins None.
Dr. Hammond None.
Dr. Kempen CBM: S; Gilead: C; NEI: S; Sight for Souls: S. In addition, the MUST Trial received a donation of fluocinolone acetonide intraocular implants from Bausch + Lomb for patients who would be unable to enroll in the trial without a donated implant.
Dr. Lim Heidelberg Engineering: L; National Healthcare Group Eye Institute, Tan Tock Seng Hospital: S; Novartis: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review