Download PDF
When Anthony Fauci, MD, urgently needed to jump-start a COVID-19 vaccine pipeline, he turned to Larry Corey, MD, an internationally renowned virologist and trusted colleague, to lead its operations. The mission was “simple”: Enroll massive, simultaneous COVID-19 vaccine trials using multiple vaccine technologies and meet a stretch goal to deliver 300 million vaccine doses by mid-2021.1
Dr. Corey oversees this national pipeline research via the COVID-19 Prevention Network, based at the Fred Hutchinson Cancer Research Center in Seattle. His approach is to “harmonize” trials toward similar metrics, so researchers can better compare apples to apples with safety and efficacy data. With novel vaccines, this harmonizing helps reveal the nuances of each candidate.
The initial national strategy: Develop two vaccine candidates in four different platforms as a hedge against potential issues with safety, efficacy, or supply lines.2 Investing in simultaneous—versus sequential—trial phases and production capacity further accelerated development.
“There’s a lot of ingenuity from the many scientists involved; it’s been really impressive,” said Steven Yeh, MD, who serves as a curator of the aao.org/coronavirus site, along with fellow ophthalmologists James Chodosh, MD, MPH, and Gary N. Holland, MD.
First to Arrive: mRNA Vaccines
COVID-19 vaccines are the first authorized mRNA (messenger RNA) vaccines for human use, although the technology has been studied for a decade. (The Johnson & Johnson vaccine will be covered in the June EyeNet.) “There have been phase 1 and 2 trials of mRNA vaccines for a variety of cancers and infections such as HIV, rabies, and Zika, and safety concerns have been nil,” said Dr. Chodosh, at Harvard and Massachusetts Eye and Ear in Boston.
Twelve-month data analyses from the clinical trials are expected this summer. In the interim, “I think there’s now a sufficient track record using this technology to say that the mRNA vaccines are safe,” Dr. Chodosh said.
Pfizer/BioNTech. Pfizer’s vaccine BNT162b2 (tozinameran; Comirnaty) earned Emergency Use Authorization (EUA) in the United States on Dec. 11, 2020. The international, combined phase 2/3 trial gave a two-dose injection, 21 days apart, of vaccine or placebo to nearly 43,500 adults age 16 years and older.2 The first dataset reported remarkable efficacy of 95% in preventing COVID-19 disease: Of those who received placebo (n = 21,728), 162 developed COVID-19, compared to eight of those who received the vaccine (n = 21,270). Similar efficacy held across age, sex, race, body mass index, and existing morbidities such as HIV and hepatitis B or C. Trial exclusions included a history of COVID-19, immunosuppressive therapy, and immunocompromising conditions.
Data on immunogenicity and duration of response were not part of the EUA application.2 Full data analyses will follow the estimated completion of phase 2/3 on Jan. 27, 2023.3
Moderna. This mRNA vaccine (mRNA-1273) was the second to earn EUA status, on Dec. 18, 2020. This phase 3 trial gave a two-dose injection, 28 days apart, of vaccine or placebo in 30,420 adults age 18 years and older (each cohort comprised 15,210 participants).4 This vaccine showed 94.1% efficacy in preventing COVID-19: All told, 185 in the placebo group developed disease, compared to 11 who received the vaccine.4
Moderna’s study, conducted with the National Institute of Allergy and Infectious Diseases, targeted participants at high risk of infection with SARS-CoV-2, severe COVID-19 disease, or both. Although participants were drawn from diverse racial and ethnic groups, ultimately 80% were White.4 The study stratified cohorts by age and risk of severe COVID-19 due to chronic lung disease, heart or liver disease, diabetes, and other conditions.4 Full data analyses will follow the estimated completion of phase 3 on Oct. 27, 2022.5 (Actual infection rates in late 2020, higher than in the initial study design of 0.75%, allowed for faster enrollment and completion for this trial.4)
 |
|
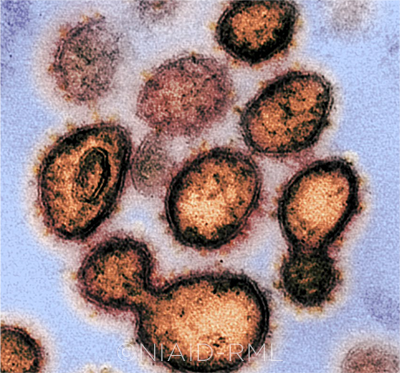
SARS-COV-2. This image shows virus particles emerging from the surface of cells cultured in the lab. The tell-tale spikes can be seen on the outer edge of the particles.
|
How mRNA Vaccines Work
The Pfizer and Moderna vaccines carry mRNA that codes for the SARS-CoV-2 spike protein. The mRNA enters the host cell’s cytoplasm, where molecules translate the mRNA’s genetic code to build the spike protein, and expresses spikes on cell surfaces.1
“The spike protein is the ligand, or molecule on the surface of the virus that binds to the host cell,” said Dr. Chodosh. “Without that first interaction, there’s no infection. Once you have antibodies to the spike protein, if you become infected, those antibodies will limit the infection.”
Because mRNA doesn’t enter the host-cell nucleus, it doesn’t affect DNA. “This is simply the message introduced into the cytoplasm of the cell and translated directly into the spike protein,” said Dr. Chodosh. “These vaccines contain very little besides the nucleic acid that codes for the spike protein.”
This may be a selling point for vaccine-hesitant patients. “Many vaccines need to add an adjuvant, a substance that lets the immune system know that foreign matter has been introduced,” said Anna Wald, MD, MPH, at the University of Washington School of Medicine in Seattle. “These mRNA vaccines are self-adjuvanting: Our bodies recognize viral strands of mRNA as foreign, and that’s enough of a danger signal for the immune cells to respond.”
Overall safety with emergency use. “Data on past vaccines is incredibly reassuring,” Dr. Wald said. She noted that a study published last summer,6 looking at 57 vaccines used for decades, “concluded that vaccines are incredibly safe and that safety signals had been detected within a few weeks” and added, “the initial COVID studies followed participants longer than two months.”
“The benefits outweigh the risks; that’s the standard for an EUA,” said Dr. Holland, at the University of California, Los Angeles (UCLA). “Reactogenicity appears far more likely than true adverse events, and fever, fatigue, and muscle soreness are expected indications that the immune system has been stimulated.”
Too risky for some patients? Questions remain vis-à-vis the use of these vaccines in children, pregnant women, and patients who have autoimmune diseases, among other vulnerable patient populations. For example, “Should people be vaccinated after a bone marrow transplant? Will there be memory cells in the stem cell transplant that protect them?” said Dr. Chodosh.
He added, “Immune-compromised people are at potentially greatest risk for severe disease and death from COVID, but will someone who has difficulties making antibodies benefit from an mRNA vaccine, or should they get a different kind of vaccine more likely to generate T-cell–mediated immunity? We don’t know the details about the immune responses to these vaccines yet.”
Efficacy versus effectiveness. As with other clinical trials, in vaccine studies, efficacy refers to the difference between the fraction of participants who develop a given disease in placebo and treatment cohorts. Effectiveness measures how well a vaccine prevents disease in the real world, beyond the controlled environment of a trial—a number often well below efficacy. “There are so many influences on effectiveness: age, other health concerns, correct vaccine administration,” said Dr. Holland. “The real-world risk of infection after vaccination may be higher than 5%.”
Immunogenicity and T cells. Knowledge of the nuances of the immune system’s response to SARS-CoV-2 or its vaccines—are T cells activated? or memory cells?—is still out of reach. One study found that asymptomatic, mild cases caused a robust, T cell–mediated immune response.7
“We don’t know to what degree these vaccines are generating immunity beyond just antibodies,” said Dr. Chodosh. “If you look at vaccine reporting, [studies] will often report either antibody responses or T cell responses—and, sometimes, both—but they don’t always know which is critical, and it’s often a combination that’s necessary.”
“We think that what’s really critical to preventing this infection are neutralizing antibodies,” said Dr. Wald. “We also think there’s a T cell response that might be important in the long run, but we don’t yet know what the correlate of immunity is.”
“Immunogenicity data show that both antibody response and cellular response are favorable for at least two months after Pfizer’s or Moderna’s two-dose protocol,” said Dr. Yeh, at Emory University School of Medicine in Atlanta. “How long this immunogenicity lasts, though, is still under investigation.”
 |
|
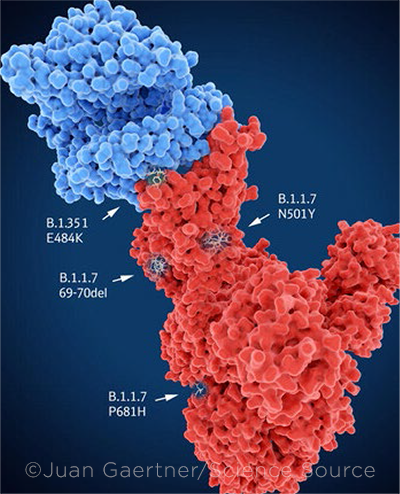
VARIANTS. Main mutation sites of selected variants. The spike protein (red) is bound to the angiotensin converting enzyme 2 (blue).
|
Hang On to Your Masks
As new vaccines enter the global pipeline through 2021, it’s tempting—although short-sighted—to give in to pandemic fatigue. “We’re in a difficult place, and until the prevalence in the community is way down, everybody has to remain vigilant,” said Dr. Chodosh. “If folks can home in on keeping themselves safe, the vaccines will have a chance to do their work, and it will look a lot better on the other side of this.”
Risks of transmission. Multiple studies have reported transmission from presymptomatic or asymptomatic carriers. “It’s not as if these antibodies are coating your nasopharynx, preventing virus from entering cells,” Dr. Chodosh said. “Both the Pfizer and Moderna vaccines appear to very effectively prevent severe disease—not by preventing infection, but by reducing replication and the ability of the virus to grow to extraordinary levels.”
“We don’t know whether you could still acquire the infection after vaccination and pass it on, so you need to stay in a mask,” said Dr. Holland, “and patients not yet vaccinated could be asymptomatically infected and place you, your staff, and other patients at risk.”
Issue of lag time. “After the mRNA injection, you have a period where the spike protein is manufactured and you’re starting to make antibodies,” said Dr. Yeh. “With Pfizer’s data, you can see that the break point, where you start to see protection offered, is around 12 days after the first dose.” This lag time opens the door to infection.
Issue of durability. Pfizer and Moderna were granted EUA after two months of data. Phase completion with two-year follow-up data is yet to come.8 “We don’t yet know about durability—whether you’ll need an annual booster or you’ll get enough memory immune response to have memory B cells that are primed and can be recruited rapidly if you see the pathogen again,” said Dr. Corey.
Hard truths about herd immunity. “Herd immunity” can be a misleading concept: It implies that immunity in other people can reduce your likelihood of disease.
“Herd immunity, on a population basis, is when you achieve a level in which so many people have been previously infected that the likelihood of new infections is reduced,” said Dr. Corey. “It doesn’t mean you’ve stopped transmission of the disease from one person to another; it just means you’ve kept the disease out of its exponential growth phase. We have no real idea, at present, if SARS-CoV-2 will be closer to measles or influenza.”
Herd immunity involves R-0 (“R-naught”). “Herd immunity is what mathematicians talk about: to decrease your R-0 to less than one, but it doesn’t mean R-0 becomes zero,” Dr. Corey said. “Mathematical calculations about herd immunity are about tackling R-0 and leaving the medical community to worry about ventilators and deaths.”
“We won’t be at a point where enough people are vaccinated to [be able to] stop mask wearing and physical distancing for a very long time, probably at least January 2022,” Dr. Wald said. “You need a very high vaccination rate, perhaps 70% to 80%.” However, she said, the exact percentage is currently unknown. “We don’t have that data yet. We don’t yet know the effect of these vaccines on onward transmission: The studies haven’t shown that they prevent asymptomatic replication of the virus in your nose, which may still leave you infectious to others.”
Last but not least: variants. SARS-CoV-2 appears to mutate about twice a month, said Dr. Corey. Mapping the evolution of the virus began with mutations in the spike protein and receptor-binding domains.9
“There are early, documented cases in which people were reinfected,” Dr. Holland said. It’s possible that these cases may have been related to waning immunity, he noted. “If you only had mild respiratory symptoms, your immunity might be less robust than if you had major organ involvement. By contrast, future mutations of the virus may allow it to evade even a strong immune response generated by previous infection or vaccination—and cause reinfection.”
The United Kingdom, South Africa, and Brazil detected the first, more transmissible variants of the virus. Because the first vaccines target the genetic blueprint for the first virus in Wuhan, new vaccines may need to evolve with the virus. “As viruses develop point mutations, it’s critical to determine which mutations are functionally significant, such as [those capable of] increasing virulence or transmission,” said Dr. Yeh.
Looking to the Future
Will hospital systems begin to require proof of COVID vaccination before entry?
“I think COVID will be in the population for some time, if not indefinitely, like the flu, where we have to get vaccinated every year,” said Dr. Chodosh. “But we’d have to have enough vaccine for everybody before we could mandate it. Maybe in 2022 we would be at a point where the FDA says we have enough data to formally approve this.”
Given the pandemic’s global scale, managing the vaccine pipeline is as challenging as the science. Challenges include developing vaccines that can be shipped to low-resource areas that don’t have adequate refrigeration. “We’re in vaccine shortage, so we need [to develop] a diversity of vaccines that can be made in large quantities and don’t have cold-chain issues,” said Dr. Corey.
Given the unknowns, should we brace ourselves for COVID-22 or -23? “This is the third cross-species transfer of a coronavirus, and there’s no reason to believe that there won’t be more,” said Dr. Corey. “The first axiom of infectious disease: Never underestimate your pathogen.”
Other Candidates to Watch
Here’s an overview of several candidates in the pipeline:
Viral-vector vaccines are made from an attenuated adenovirus (common cold virus).
Oxford/AstraZeneca’s candidate uses a nonreplicating chimp adenovirus to carry genetic code from SARS-CoV-2 into human cells’ nuclei and make the mRNA to build the spike protein.
In a pooled interim analysis of four international trial sites, the vaccine (AZD1222; ChAdOx1) showed 70.4% efficacy in preventing COVID-19 disease after two doses, with 64.1% after one dose.1 Their two-dose vaccine appears more efficacious when the first dose is half-strength, although the initial trial only gave the half-dose to participants younger than age 55.1 The vaccine was approved in the United Kingdom and is being distributed in European Union countries. Estimated phase 3 completion: Feb. 21, 2023.2
Johnson & Johnson/Janssen’s candidate uses adenovirus 26 (Ad26) to carry genetic code from the Wuhan strain of coronavirus to build spike proteins. The Ad26 vaccine was used for Ebola and HIV in Africa, although global success of an adenovirus vaccine depends on prior regional exposure to that adenovirus.
Interim results from the combined phase 1/2a study showed that this candidate produced neutralizing antibodies in at least 90% of participants on day 29 after the first dose, and in 100% by day 57.3 This trial is comparing five combinations of low- and high-dose and single- and double-dose injections.3 Estimated phase 3 completion: May 11, 2023.4
The FDA issued EUA for the single-shot vaccine on Feb. 27. Johnson & Johnson has teamed with Merck to ramp up production.
Protein-based vaccines use a protein subunit or shell to stimulate immune response.
Novavax is testing its candidate (NVX-CoV2373) in 30,000 diverse, high-risk participants with comorbidities, to complete Dec. 30, 2022.5
Sanofi/GSK is testing its candidate with various adjuvants in 440 participants, to complete in October 2021.6
Monoclonal antibodies, used to treat early COVID-19, are being studied for prevention.
Regeneron is testing a combination of two antibodies (REGN10933 and REGN10987) in about 2,000 adults.7
Eli Lilly is testing its antibody (LY3819253) with about 2,400 staff and residents in nursing and assisted living facilities.8
Coronavirus-based vaccines, such as Sinovac’s vaccine in China, use inactivated SARS-CoV-2 virus.
___________________________
1 Voysey M et al. The Lancet. 2021;397(10269):99-111.
2 www.clinicaltrials.gov; NCT04516746.
3 Sadoff J. et al. N Engl J Med. Published online Jan. 13, 2021.
4 www.clinicaltrials.gov; NCT04614948.
5 www.clinicaltrials.gov; NCT04611802.
6 www.clinicaltrials.gov; NCT04537208.
7 www.clinicaltrials.gov; NCT04452318.
8 www.blaze2study.com. Accessed Feb. 5, 2021.
|
___________________________
1 Slaoui M, Hepburn M. N Engl J Med. 2020;383(18):1701-1703.
2 Polack FP et al. N Engl J Med. 2020;383(27):2603-2615.
3 www.clinicaltrials.gov; NCT04368728.
4 Baden LR et al. N Engl J Med. 2021;384(5):403-416.
5 www.clinicaltrials.gov; NCT04470427.
6 Tau N et al. Ann Internal Med. 2020;173(6):445-449.
7 Sekine T et al. Cell. 2020;183(1):158-168.
8 Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/vaccines. Accessed Feb. 5, 2021.
9 Greaney AJ et al. www.bioRxiv.org/content/10.1101/2020.12.31.425021v1.full.pdf. Accessed Feb. 5, 2021.
Meet the Experts
James Chodosh, MD, MPH Edith Ives Cogan Professor of Ophthalmology at Harvard Medical School and associate director of the Infectious Diseases Institute at Massachusetts Eye and Ear, Boston. Relevant financial disclosures: None.
Larry Corey, MD Coprincipal Investigator in Vaccines for the COVID-19 Prevention Network. He is also professor in the divisions of Vaccines and Infectious Diseases, Clinical Research, and Public Health Sciences at Fred Hutchinson Cancer Research Center; and professor in the departments of Medicine and Laboratory Medicine at the University of Washington, Seattle. Relevant financial disclosures: None.
Gary N. Holland, MD Professor of ophthalmology, the Jack H. Skirball Chair in Ocular Inflammatory Diseases, and director of the Department of Ophthalmology Clinical Research Center at UCLA. He also is a member of the UCLA Stein Eye Institute. Relevant financial disclosures: None.
Anna Wald, MD, MPH Head of the Division of Allergy and Infectious Diseases and professor in the departments of Medicine, Allergy and Infectious Diseases, Epidemiology, and Laboratory Medicine and Pathology at the University of Washington, Seattle. She is also professor in the Vaccine and Infectious Diseases Division at the Fred Hutchinson Cancer Research Center. Relevant financial disclosures: None.
Steven Yeh, MD Professor of ophthalmology at the Emory Eye Center and Emory University School of Medicine in Atlanta. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chodosh British Journal of Ophthalmology: E; FDA: C; NEI: S.
Dr. Corey None.
Dr. Holland Childhood Arthritis and Rheumatology Research Alliance: S; NEI: S; University of California Firearm Violence Research Center: S.
Dr. Wald NIH: S.
Dr. Yeh Santen: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|