By Anat Galor, MD, Bennie H. Jeng, MD, and Careen Y. Lowder, MD, PHD
Edited Thomas A. Oetting, MD
This article is from January 2007 and may contain outdated material.
John Roberts* felt like one of his biggest fears was coming true—“I don’t even like needles, and now I’m told that I have to go under the knife.” Eight weeks earlier he had noticed fluctuations in his vision that progressed to permanently decreased vision in both eyes. His ophthalmologist diagnosed bilateral corneal edema, started him on prednisone and, to Mr. Roberts’ alarm, recommended corneal transplantation. Despite the medication (60 mg per day orally), Mr. Roberts didn’t notice any improvement in his vision. This, together with his fear of surgery, prompted him to seek a second opinion.
We Get a Look
When we saw Mr. Roberts, we noted that he was a 57-year-old Caucasian whose medical history included a transient episode of diplopia in 1984 and a diagnosis of multiple sclerosis in 1999. In addition to the prednisone, he was taking hydrochlorothiazide, bupropion, terazosin, ranitidine, amantadine, baclofen, gabapentin and interferon beta.
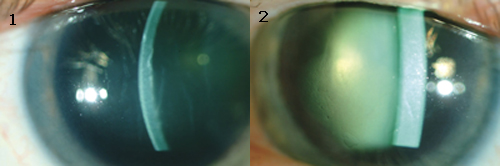
His BCVA was 20/70 in the right eye and 20/100 in the left eye. Pupillary examination, extraocular movements and IOPs were all within normal limits. A slit-lamp exam revealed bilateral stromal edema with microcystic edema, but without obvious guttata (Figs. 1 and 2). His pachymetry readings were 838 µm for the right eye and more than 1,000 µm for the left. The dilated fundus examination was unremarkable.
The Differential Diagnosis
Causes of corneal edema include endothelial disorders, inflammatory processes, ocular surgery, trauma and toxins.
Endothelial disorders. Fuchs’ endothelial dystrophy is the most common cause of corneal edema in our patient’s age group. It is an inherited condition resulting in the gradual loss of endothelial cells. Fuchs’ affects both eyes and is slightly more common among women than men. It generally begins during the fifth decade of life and gradually progresses. Symptoms include decreased vision, which is worse upon awakening, and pain secondary to ruptured bullae. Clinical findings include endothelial guttata, Descemet’s membrane folds, stromal edema and epithelial edema. With severe corneal edema, it is often difficult to appreciate the endothelial changes.
A less common endothelial dystrophy is posterior polymorphous corneal dystrophy, a disease affecting younger patients. It is characterized by isolated or coalesced posterior corneal vesicles and a bandlike configuration of Descemet’s membrane with scalloped edges. Histologically, the endothelial cells look like epithelial cells and are multilayered with microvilli. Our patient had no obvious guttata or posterior corneal vesicles on examination.
Chandler’s syndrome, a subset of iridocorneal endothelial syndrome, is a slowly progressive disorder that can cause corneal edema. It is found unilaterally in young to middle-aged patients; it affects women more often than men. The underlying abnormality is an epithelial-like endothelial layer, which proliferates and leads to corneal edema, iris abnormalities and glaucoma.
|
What's Your Diagnosis?
|
 |
|
AT THE SLIT LAMP. We found that Mr. Roberts had bilateral stromal and microcystic edema, with his left eye (2) worse than his right (1).
|
Inflammatory processes. Several inflammatory processes can lead to corneal edema, the most common being endotheliitis associated with herpes- viruses. Endotheliitis is an immune reaction that can occur months to years after an episode of infectious keratitis. Symptoms include pain, photophobia and redness. Clinical features include keratic precipitates (KP), stromal edema and iritis. Unlike interstitial keratitis, endotheliitis is not associated with stromal infiltrates or neovascularization. The three main types of endotheliitis include disciform endotheliitis, which presents as an area of central stromal edema overlying an area of KP; diffuse endotheliitis, which presents with diffuse KP and edema; and linear endotheliitis, which presents with linear KP and corneal edema localized to the peripheral cornea. With Mr. Roberts, we found no evidence of an inflammatory process such as keratitis or uveitis.
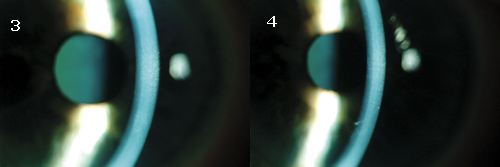 |
| <SURGERY IS NOT NEEDED. Much to Mr. Roberts' relief, the edema resolved in both his right (3) and left (4) eyes. |
Ocular surgery. This can lead to corneal edema immediately after surgery or years later. Causes of acute postop corneal edema include endothelial damage due to ultrasound energy, infusion of toxic substances into the anterior chamber and stripping of Descemet’s membrane. Patients with underlying corneal endothelial dysfunction are at higher risk for endothelial damage, even after uncomplicated intraocular surgery. Pseudophakic bullous keratopathy (PBK) can manifest itself years after cataract surgery and is a result of gradual loss of endothelial cells after surgery in addition to acute perioperative endothelial damage. Although the incidence of PBK has decreased as surgical techniques and lens designs have improved, it is still an important cause of visual disability following routine and complicated cataract surgery. Clinical symptoms include poor vision, pain, foreign body sensation and photophobia. Clinical findings include stromal and epithelial edema, as well as folds in Descemet’s membrane. Our patient denied a history of ocular surgery or trauma, and there was no evidence of other anterior segment abnormalities.
Toxic insult. Several substances have been reported to cause endothelial dysfunction after intraocular, topical and systemic administration. Benzalkonium chloride, a preservative found in many topical eyedrops and topical anesthetic agents, has caused corneal edema after inadvertent intraocular use. Corneal edema after local contamination with chlorhexidine (Hibiclens keratopathy) is a well-known clinical entity. In addition, amantadine is a systemic medication that has been associated with corneal edema. In view of Mr. Roberts’ history and physical findings, we considered a toxic insult as a potential cause of his corneal edema.
Treatment
Given the association between amantadine and corneal edema, and with the approval of the patient’s neurologist, the patient was instructed to discontinue taking amantadine.
Complete resolution of the edema was noted after two weeks; this corresponded to improved visual acuities of 20/25 in the right eye and 20/30 in the left, as well as improved pachymetry measurements of 601 µm in the right eye and 616 µm in the left (Figs. 3 and 4).
Discussion
Amantadine is a medication used to treat fatigue associated with multiple sclerosis. Ocular side effects include visual loss, hallucinations, oculogyric crises and mydriasis.1,2 Reported corneal side effects include diffuse, white punctate subepithelial opacities, which can be associated with superficial punctate keratitis, epithelial edema and decreased vision. These deposits usually occur one to two weeks after initiation of therapy and resolve within a few weeks after cessation of treatment.2,3
Amantadine-induced corneal edema has been described in two previous case reports.4,5 Unlike our patient, who had been taking amantadine since 1999, the corneal edema in these patients began soon after initiation of treatment. All three cases of edema resolved promptly on discontinuation of therapy.
Our case highlights the importance of considering medication toxicity in a patient with corneal edema and no obvious ocular cause. Making a correct diagnosis saved Mr. Roberts from undergoing unnecessary surgery.
_____________________________
* Patient name is fictitious.
_____________________________
1 Postma, J. U. and W. Van Tilburg. J Am Geriatr Soc 1975;23:212–215.
2 Fraunfelder, F. T. and S. M. Meyer. Am J Ophthalmol 1990;110:96–97.
3 Nogaki, H. and M. Morimatsu. J Neurol 1993;240:388–389.
4 Hughes, B. et al. Cornea 2004;23:823–824.
5 Blanchard, D. L. Cornea 1990; 9:181.
_____________________________
All the authors are affiliated with the Cole Eye Institute in Cleveland. Dr. Galor is chief resident, Dr. Jeng is with the department of cornea and external disease, and Dr. Lowder is with the uveitis department.