By Siehyean Kiew, MD, and Daniel S.W. Ting, MD, PhD
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Retinal vein occlusion (RVO) has a prevalence of 0.5%, making it the second most-common retinal vascular disorder after diabetic retinopathy.1 RVO is classified according to the anatomic level of the occlusion, with 3 major distinct entities:
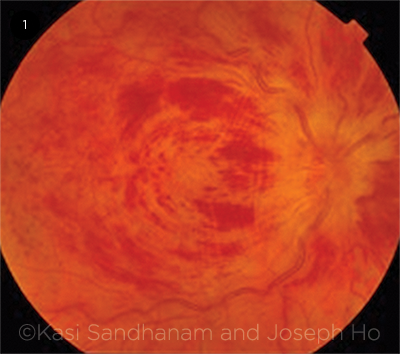
- Central retinal vein occlusion (CRVO): occlusion of the central retinal vein at the level of, or posterior to, the lamina cribrosa (Fig. 1)
- Hemiretinal vein occlusion (HRVO): occlusion at the disc, involving either the superior or inferior hemiretina
- Branch retinal vein occlusion (BRVO): occlusion of a tributary vein, typically at the site of an arteriovenous crossing; thought to be caused by compression from an overlying atherosclerotic arteriole
This article will focus on diagnosis and management of the first entity, CRVO.
Risk Factors
Systemic disorders. Systemic risk factors for CRVO include increasing age, diabetes mellitus, and hypertension. In selected cases, hypercoagulable states, including hyperhomocysteinemia and factor V Leiden mutation, or local vessel factors such as vasculitis are also associated with increased risk of CRVO. The literature also contains case reports of many other systemic conditions possibly associated with the development of CRVO.
Ocular conditions. Open-angle glaucoma is a major ocular risk factor for CRVO.
In addition, individuals with CRVO in 1 eye are at higher risk of developing CRVO in the fellow eye.2 In the Central Vein Occlusion Study (CVOS), 4% of patients presented with bilateral CRVO at study enrollment, and a further 5% had evidence of previous CRVO in the fellow eye at baseline. In the remaining subjects, 1.4% developed CRVO in the fellow eye during 3 years of follow-up.
Other ocular risk factors include retrobulbar external compression of the central retinal vein, as occurs in thyroid orbitopathy, or compression by intraorbital space-occupying lesions.
Clinical Presentation
Patients with CRVO typically present with a history of unilateral acute, painless visual loss. Visual impairment may be severe, ranging from better than 20/40 to worse than 20/200. A relative afferent pupillary defect may be present in the affected eye.
Fundus findings. Dilated fundus examination reveals unilateral disc swelling with peripapillary intraretinal hemorrhages, dilated tortuous veins, and intraretinal dot, blot, and flame hemorrhages in all quadrants, resulting in the classic “blood and thunder” fundus appearance (Fig. 1). The macula may be edematous.
In less severe cases, disc swelling may be absent. In subacute or late presentations in which disc swelling has resolved (with or without collateral vessel formation), the flame-shaped hemorrhages clear first, leaving deeper dot/blot hemorrhages that may be difficult to distinguish from a severe microangiopathic retinopathy such as diabetic retinopathy (Fig. 2). Fluorescein angiography (FA) may help to confirm the diagnosis of CRVO.
Other key aspects. As part of the examination, the clinician should note the intraocular pressure and cup-to-disc ratio, which may suggest concurrent glaucoma, as well as any sequelae, such as rubeosis iridis. Undilated gonioscopic examination is important to rule out neovascularization of the angles.
 |
|
ACUTE CRVO. Classic “blood and thunder” fundus appearance of a patient presenting acutely with central retinal vein occlusion of the right eye.
|
Types of CRVO
Clinically, CRVO may be divided into 2 major subtypes: ischemic and nonischemic.
Ischemic. The CVOS investigators defined ischemic CRVO as evidence of more than 10 disc areas of capillary nonperfusion on 7-field fundus FA (although investigators are reassessing this definition in light of recent advances in widefield angiography).
Ischemic CRVO may be identified by the following characteristics:
- Poor visual acuity (>90% had VA of <20/200)
- Presence of a relative afferent pupillary defect in the affected eye
- Presence of extensive dark, deep intraretinal hemorrhages
- Presence of multiple cotton-wool spots
- Greater than 10 disc areas of retinal capillary nonperfusion on 7-field FA
- Reduced b-wave amplitude, reduced b:a ratio, and prolonged b-wave implicit time on electroretinography
In ischemic CRVO, visual acuity remains poor, often decreasing further over time. Causes of visual loss include chronic macular edema, macular ischemia, peripheral/global ischemia with secondary vitreous hemorrhage, and neovascular glaucoma.
Approximately 23% of eyes with ischemic CRVO develop iris neovascularization over 15 months; in the CVOS, 44% of eyes that presented with vision worse than 20/200 subsequently developed iris neovascularization.2 Some patients may develop retinal neovascularization.
Nonischemic. In the CVOS, 34% of eyes that initially presented with nonischemic CRVO underwent conversion to an ischemic perfusion status over 3 years2; conversion is heralded by rapid visual deterioration in the affected eye. Sudden decrease in visual acuity in a patient with existing nonischemic CRVO should, therefore, prompt further assessment for development of ischemic CRVO.
Of the eyes that remained nonischemic, approximately 30% showed resolution of macular edema within 15 months. Occurrence of subsequent neovascular complications is rare in nonischemic eyes.
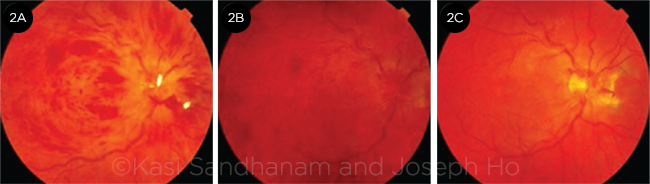 |
CHANGES OVER TIME. Same eye as shown in Fig. 1 at (2A) 1 month, (2B) 4 months, and (2C) 1 year following initial presentation, demonstrating evolution of the clinical picture. Disc edema resolves first, then the flame hemorrhages, and finally the dot and blot hemorrhages, with development of collateral vessels at the optic disc.
|
Workup
A thorough initial workup can provide useful information to guide clinical decision making.
Optical coherence tomography. OCT is useful to confirm and quantify the severity of macular edema, assess the integrity of the ellipsoid zone/photoreceptor layers, and monitor response to treatment. In clinical practice, OCT measurements often guide treatment decisions.
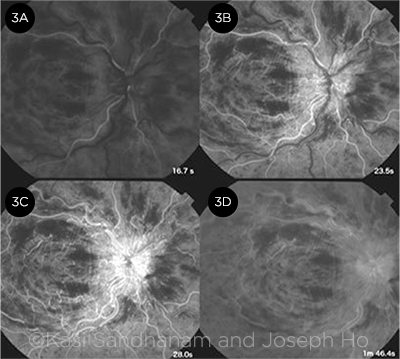
Fluorescein angiography. Features of CRVO on FA include delayed arm-to-retina time, prolonged arteriovenous transit time (markedly so in ischemic CRVO), late staining along vessel walls, capillary dropout with pruning of the vessels in areas of ischemia, and late leakage in a petalloid pattern in the presence of macular edema (Fig. 3).
Clinically, FA allows evaluation of the extent of capillary nonperfusion and the degree of macular ischemia and enables differentiation between collateral vessels and new vessels.
Systemic. Systemic evaluation is often performed in patients with CRVO and is directed by the patient’s age, coexisting risk factors, and medical history. Assessment should be performed in conjunction with an internist, as patients with RVO may be at higher risk of cardiovascular disease and cerebrovascular accidents.
There are no clear guidelines on systemic testing, but it generally begins with a dilated funduscopic examination in clinic, along with a detailed medical history to identify risk factors; further assessment includes blood pressure and serum glucose, complete blood count, and erythrocyte sedimentation rate. In young patients without clear risk factors, additional testing should be considered to exclude a hematologic or vasculitic etiology.
 |
|
FLUORESCEIN FINDINGS. FA at 4 time points shows (3A) masking from intraretinal hemorrhages, (3B) delayed arteriovenous transit time, (3C) leakage at the swollen optic disc, and (3D) late staining of the vessel walls.
|
Treatment
All patients should optimize control of systemic risk factors, with the help of their internist. Management of the ocular manifestations may be divided into the following areas.
Macular edema. Both laser and medical therapies have been used in the treatment of macular edema.
Laser. Studies assessing grid-pattern laser photocoagulation for treatment of macular edema in CRVO showed anatomic improvement without improvement in visual acuity.2
Anti–vascular endothelial growth factor. Intravitreal anti-VEGF therapy is currently the gold standard of treatment for macular edema associated with CRVO. There is increasing evidence that anti-VEGF therapy results in lower risk of visual loss, higher rates of visual gains, greater reduction in central retinal thickness, and reduced risk of progression to iris neovascularization.
For example, the CRUISE study reported that intravitreal ranibizumab significantly improved best-corrected visual acuity (BCVA) at 6 and 12 months compared with sham injections. In the open-label extension HORIZON trial, the eyes initially treated with sham and subsequently treated with ranibizumab showed improvement in BCVA but did not catch up to the visual outcomes attained by the group that received ranibizumab at enrollment. This finding suggests that delaying treatment for macular edema has adverse effects on visual outcomes.
Aflibercept, a VEGF-trap molecule, has also been shown to improve BCVA compared with sham and laser treatment in the COPERNICUS and GALILEO trials.
More recently, SCORE2, a randomized noninferiority trial including eyes with CRVO or HRVO, demonstrated that bevacizumab was noninferior to aflibercept in terms of visual acuity gain at month 6 compared to baseline (mean improvement of 18.6 vs. 18.9 ETDRS letters, respectively).3
Corticosteroids. Corticosteroids reduce retinal capillary permeability and inhibit the expression and metabolic pathway of VEGF. The SCORE-CRVO trial demonstrated that intravitreal triamcinolone acetonide was superior to observation for visual loss associated with CRVO-related macular edema. The GENEVA trial evaluated the use of a sustained-release intravitreal dexamethasone implant (Ozurdex) and demonstrated improvements in visual acuity and macular thickness compared with both sham and laser-treated groups.
More recently, the Clinical Efficacy and Safety of Ranibizumab Versus Dexamethasone for Central Retinal Vein Occlusion (COMRADE C) trial compared intravitreal ranibizumab 0.5 mg (monthly for at least 3 months, followed by as-needed dosing) to a single injection of Ozurdex. This trial reported similar efficacy between ranibizumab and Ozurdex but found a higher incidence of adverse effects in the group receiving Ozurdex.
Retinal ischemia. Current evidence recommends regular monitoring of patients with ischemic CRVO for development of iris or angle neovascularization, for which panretinal laser photocoagulation (PRP) remains the mainstay of treatment.
There is currently no evidence to recommend prophylactic treatment prior to the development of new vessels. However, in circumstances where regular follow-up is impractical and the degree of ischemia is severe (high risk of progression to neovascularization), prophylactic PRP may be appropriate.
Anti-VEGF agents are antiangiogenic and may be useful adjuncts to PRP in the management of patients with CRVO and associated anterior segment neovascularization, particularly when the view of the fundus is not sufficiently clear to permit adequate PRP.
Venous outflow. A number of alternative therapies focused on improving retinal blood flow have been described. These include the use of antiplatelet agents (e.g., ticlopidine),4 hemodilution,5 and thrombolytic agents delivered systemically, intravitreally, or directly into a retinal vein during pars plana vitrectomy.
Techniques to alleviate a possible compartment syndrome, with optic nerve sheath decompression through an orbital approach or radial optic neurotomy via a pars plana approach, have been tried. However, these are no longer used because of their limited benefit and significant risks.
Creation of a laser chorioretinal venous anastomosis (L-CRA) to bypass the occluded central retinal vein has been reported to be beneficial in nonischemic CRVO, with improvement in visual acuity and reduced rates of ischemic progression,6 but less so in eyes with the ischemic type of disease. The failure of anastomosis was most likely due to endothelial cell damage secondary to ischemia.7
___________________________
1 Klein R et al. Arch Ophthalmol. 2008;126(4):513-518.
2 Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115(4):486-491. [Erratum in Arch Ophthalmol. 1997;115(10):1275.]
3 Scott IU et al. JAMA. 2017;317(20):2072-2087.
4 Yamamoto T et al. Am J Ophthalmol. 2004;138(5):809-817.
5 Wolf S et al. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):33-39.
6 McAllister IL et al. Ophthalmology. 2010;117(5):954-965.
7 Kwok AK et al. Br J Ophthalmol. 2003;87(8):1043-1044.
___________________________
Dr. Kiew is an ophthalmology resident at the Singapore National Eye Centre. Dr. Ting is an associate consultant at the Singapore National Eye Centre and assistant professor at Duke-National University Singapore. Relevant financial disclosures: None.