By Tyler F. Gorman and Syril Dorairaj, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
The first clinical report of optic disc hemorrhage (DH) is credited to Jannik P. Bjerrum in 1889. He detailed a number of glaucoma patients whose eyes had elevated intraocular pressure (IOP) and bleeding within the optic nerve head and retina. It was not until 1970, however, that Dr. Stephen Drance and Dr. Ian Begg suggested that DH was an important marker of glaucomatous damage.
Few clinical findings are as controversial or as poorly understood as DH in glaucoma patients. DH has been reported to be a risk factor for the onset1 and progression2,3 of glaucomatous optic neuropathy. Recently, many studies have been conducted to better understand the etiology and prognostic value of DH in eyes with glaucoma, but no definitive answers have yet been established. Most researchers would agree, however, that a DH in a glaucomatous eye is a negative prognostic factor and, in most cases, indicates advancing damage to the retinal nerve fiber layer (RNFL).1-4
What Is a Disc Hemorrhage?
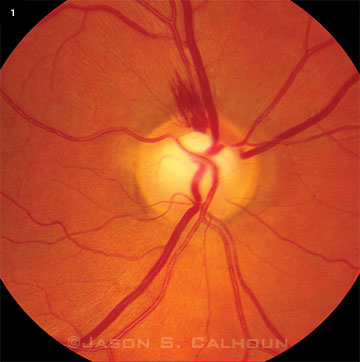
A typical DH in an eye with glaucoma appears as a splinter-like or flame-shaped area of bleeding, usually with feathered ends and oriented perpendicular to the disc margin (Fig. 1). The most common location of DH is at the temporal aspect of the disc. Disc hemorrhages tend to be small and extend from within the RNFL of the optic disc into the peripapillary region. DH is often associated with notching and structural change in the optic disc rim, focal defects of the RNFL, progressive defects of the visual field (VF), and beta zone peripapillary atrophy (βPPA). DHs are rarely found in normal eyes, but they are detected in approximately 4 to 7 percent of eyes with glaucoma.5 Of this minority of glaucoma patients who manifest DH, there are some who tend to bleed repeatedly.
 |
|
IN-DEPTH EXAMINATION. A DH is visible on the superior aspect of the optic disc. Notice the thinning of retinal nerve fiber layer and notching of the rim, which are structural risk factors associated with DHs.
|
Why Is DH Important?
Although some researchers believe that DH is unrelated to VF progression, others say it is a convincing prognostic factor for the onset or progression of VF loss.
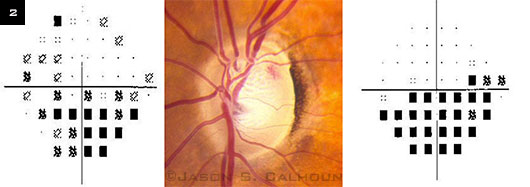
Siegner and Netland6 found that there is an increased risk of structural and functional damage to the optic disc in glaucomatous eyes with a DH, as 63 percent of visual fields and 79 percent of optic discs showed progressive changes after the appearance of a DH (Fig. 2). Likewise, Ishida et al.2 reported that out of 32 eyes with DH, 26 (81.3 percent) showed progressive losses in VF, with a mean follow-up of 5.6 years. However, not all eyes demonstrated progressive VF defects after a DH was discovered.
DHs are also important because studies have shown that eyes with ocular hypertension that develop a DH are six times more likely to progress to primary open-angle glaucoma than hypertensive eyes without DHs.1 Based on these studies, it is clinically important to understand the rate at which damage occurs after a DH, and which eyes are prone to progress more rapidly.
Pathophysiology
The process underlying DH development remains unclear, but many studies have suggested that DH is the result of vascular events within the neuroretinal tissue, which lead to nerve fiber loss. Another hypothesis suggests that the rapid deterioration of the rim tissue and development of rim notching causes stress on the microvasculature, resulting in DH.2
Structural loss. Many researchers have focused on identifying the temporal relationships between the onset of DH and the structural and functional losses in the optic nerve that cause it. De Moraes and coworkers3 recently suggested that DH is the result of ongoing structural loss, as 90 percent of DHs found were in areas of identified rim notching.
Likewise, Law and colleagues4 viewed a number of optic disc photographs before and after the onset of DH and found that 100 percent of the eyes that later developed DH had prior rim notching in the same location or adjacent to the DH. These observations reinforce the theory that structural features within areas of rim notching may eventually lead to a DH. Some researchers now hypothesize that DH is caused by the loss of rim scaffolding that supports the involved vascular tissue as well as stress on the blood vessels due to neurodegeneration.
Making the connection. It is also possible that a combination of ischemia and structural collapse leads to DH. Hypothetically, an initial ischemic insult could cause a first hemorrhage, which leads to rapid VF damage. Ongoing degeneration of vascular and neuroretinal tissues as well as microvascular occlusion of the blood supply to the disc may lead to recurrent DH. Additional longitudinal studies need to be conducted to determine the actual pathophysiology of DH.
 |
|
DH AND VISUAL FIELD. Progressive changes of visual field in the left eye due to disc hemorrhaging. Four months elapsed between the visual field shown at the left and the one at the right. Inferior nasal visual field loss corresponds to the presence of a superior temporal disc hemorrhage.
|
Diagnosis
Various imaging devices have been developed to allow objective evaluation and measurement of the optic nerve and RNFL. These advancements have improved the ability to detect and follow glaucoma. However, DHs are very small and may be difficult to detect during a clinical exam, and none of the present imaging devices reveal them consistently. In fact, the Ocular Hypertension Treatment Study showed that they are overlooked in many examinations. Of the 128 eyes with DH, 21 (16 percent) were detected by both clinical examination and review of fundus photography, while 107 (84 percent) were detected solely by review of photography.1
Fundus examination, preferably with optic disc photography, must be done both carefully and frequently if these hemorrhages are to be reliably detected. Improving identification of DH is important, as aggressive management of eyes with glaucoma with DH may be warranted due to the suspected increased risk of associated disease progression.1
Improving detection. Often, DHs occur in areas of previous damage, such as an area with an RNFL defect or rim notching. Usually, the highest diagnostic yield is gained by focusing on the temporal half of the disc. DHs are usually present at the edge of the optic disc or adjacent to prior rim notching or RNFL defect and frequently arise near βPPA. To minimize the number of overlooked DHs, the examiner must make a particular effort to seek them.
Risk factors. Given that the pathogenesis of a DH is not entirely understood, recognizing risk factors linked with DH may provide a better understanding of the mechanisms in its occurrence. Studies have identified a number of associated risk factors, including thinner neuroretinal rim and notching at the baseline, as well as systemic factors such as a history of migraine, low systemic blood pressure, low mean ocular perfusion pressure, and use of systemic beta-blockers. Overtreatment of systemic hypertension may lead to complications, including stroke and reduction of ocular perfusion pressure.
Jonas et al.5 demonstrated morphological prognostic features related to the occurrence of DH and found that a smaller neuroretinal rim and larger βPPA were indicative of developing DH. The Early Manifest Glaucoma Trial found that female gender, myopia, and lower baseline or follow-up IOP were independent risk factors for DH.7
More recently, Furlanetto et al. analyzed IOP-independent and systemic risk factors for detection of DH in Low-Pressure Glaucoma Treatments Study patients and found significant associations with migraine, baseline narrower neuroretinal rim, low systolic blood pressure and mean arterial ocular perfusion pressure, and use of systemic beta-blockers.8 These studies underscore the importance of IOP-independent factors in the pathogenesis of DH in glaucoma patients.
Differential diagnosis. There is a wide range of differential diagnoses for DHs, and they may not be related to glaucoma if vitreous detachment, diabetic or hypertensive retinopathy, areas of hyperpigmentation around the disc, and neovascularization are present. All of these may be incorrectly diagnosed as an isolated DH without careful examination and the exclusion of other potential causes. Therefore, it is crucial to conduct a thorough and complete ophthalmic examination to exclude other causes before attributing DH to glaucoma.
Management
Management is not directed at the DH per se, but rather at the underlying glaucoma. Treatment of DH has proved to be very difficult in clinical trials, as many therapies have shown no correlation with reducing the frequency of DHs. However, in an observational cohort study, Medeiros et al.9 found an association between IOP-lowering therapy and VF progression in eyes with DH followed for an average of 8.2 years. They reported a beneficial effect of treatment in slowing the velocity of VF loss.
Conclusion
Multiple studies have shown that DHs are associated with glaucomatous damage and a high risk of disease progression. Identifying DHs is important because they drive relevant clinical decisions in glaucoma management to preserve visual function. However, it is uncertain whether treatment is beneficial in reducing future disc hemorrhages.
It is vital to examine the disc carefully and look for hemorrhages at every visit. This can help to identify where structural (optic disc) and functional (visual field) changes may occur, or have already occurred, and may give us a better understanding of the course of glaucoma.
___________________________
1 Budenz DL et al. Ophthalmology. 2006;113(12):2137-2143.
2 Ishida K et al. Am J Ophthalmol. 2000;129(6):707-714.
3 De Moraes CG et al. Invest Ophthalmol Vis Sci. 2009;50(10):4727-4733.
4 Law SK et al. Am J Ophthalmol. 2001;132(3):411-413.
5 Jonas JB et al. Invest Ophthalmol Vis Sci. 2002;43(9):2956-2961.
6 Siegner SW, Netland PA. Ophthalmology. 1996;103(7):1014-1024.
7 Bengtsson B et al; Early Manifest Glaucoma Trial Group. Ophthalmology. 2008;115(11):2044-2048.
8 Furlanetto RL et al. Am J Ophthalmol. 2014 Feb 7. [Epub ahead of print.]
9 Medeiros FA et al. Ophthalmology. 2010;117(11):2061-2066.
___________________________
Mr. Gorman is a student research intern, and Dr. Dorairaj is an assistant professor of ophthalmology and a glaucoma and anterior segment surgeon; both are at Mayo Clinic in Jacksonville, Fla. The authors report no related financial interests.