Download PDF
Dry eye disease, sometimes referred to as dysfunctional tear syndrome, is often a source of frustration for clinicians and patients alike. It can be progressive with major consequences for a patient’s vision and quality of life, yet it remains notoriously underappreciated, misdiagnosed, and undertreated. Approximately 20 million people in the United States (344 million people worldwide) have dry eye disease (DED), and that number is growing in both young and old adults,1 making it imperative that clinicians figure out how best to treat it.
The explosion of new information over the past 2 decades has only made DED management increasingly complex. Researchers now know, for example, that there can be a lack of correlation between signs and symptoms, a multifaceted etiology, and an overlap among symptoms of different subtypes. A lack of standardized terminology undercuts the strength of the research, and clinicians remain thwarted by a poorly understood pathophysiology, a limited range of diagnostic tests, and few treatment options that are currently approved by the U.S. Food and Drug Administration.
“We have to do better,” said dry eye expert Anat Galor, MD, MSPH, at Bascom Palmer Eye Institute in Miami.
 |
|
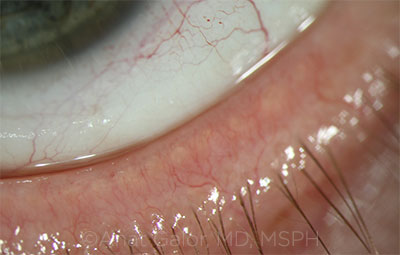
COMORBIDITY. Telangiectasias of the lid margin in a patient with rosacea.
|
Defining Dry Eye
The recent report of the Tear Film & Ocular Surface Society Dry Eye Workshop II (TFOS DEWS II), published in 2017, was a major collaborative achievement.2 Perhaps its greatest contribution, according to Deborah S. Jacobs, MD, at Massachusetts Eye and Ear’s Cornea Service in Boston, is its definition of dry eye, which was heavily debated, right down to the word order.
The end result: “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”2
Highlighting homeostasis. The disruption of homeostasis, whether in the tear film, anatomy, or the nervous system, is the unifying characteristic of all DED subtypes. “The goal of diagnosis is to pinpoint where homeostasis has been disrupted, and the goal for therapies is to restore that homeostasis,” said Dr. Jacobs.
It sounds straightforward, but the masses of data presented in DEWS II are overwhelming, making it anything but simple for clinicians to chart a course from diagnosis through treatment. “The fact that DEWS II is exponentially longer than DEWS I is a clue that we’re not getting clarity,” Dr. Jacobs said. “We just don’t have a sound understanding of the underlying pathophysiology of dry eye syndrome, and clarity will elude us until we do.”
Acknowledging neuropathic pain. DEWS II incorporates the understanding that dry eye can be a pain syndrome. “Some patients come in complaining of severe dry eye symptoms, and you look at their cornea and there’s nothing there,” said Dr. Jacobs. “We call it ‘pain without stain.’” The problem may stem from nerves injured by trauma or refractive surgery or from a systemic disease (see “Is Neuropathic Pain Involved?” below).
Is Neuropathic Pain Involved?
“When there is a major disconnect between signs and symptoms, I suspect a component of neuropathic pain,” said Dr. Galor, who then looks for a symptom profile that involves burning and pain associated with wind and light, and for signs such as persistent pain after anesthesia.
Dr. Galor also looks for comorbidities associated with neuropathic pain, such as migraine, fibromyalgia, and low back pain. “Pain doesn’t exist in isolation. Patients with other types of chronic pain sometimes suffer from chronic ocular pain as well,” she said. Lastly, Dr. Galor asks about depression and anxiety. These are common in her patients with chronic ocular pain.
The Pain and Sensation section of DEWS II doesn’t have a therapeutics section because there is a paucity of data, and the treatments don’t relate to other therapies for dry eye, Dr. Jacobs explained. But keeping corneal neuralgia under the umbrella term of dry eye makes sense because nerves are part of the system, she said. “When nerves function properly, we have homeostasis,” she said. “When they don’t, we have symptoms.”
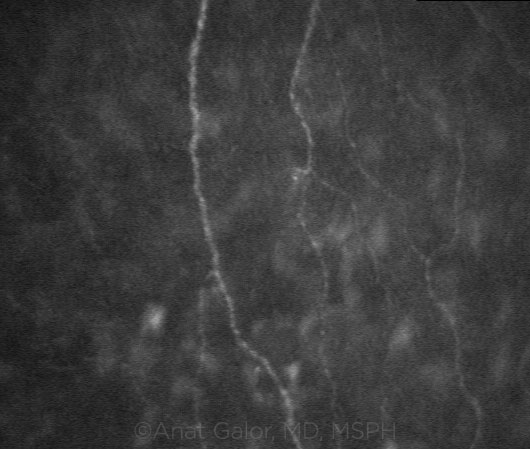
NERVE INVOLVEMENT. Confocal scanning demonstrating corneal nerves in a patient with dry eye.
|
Patient Assessment: Where to Begin
“Ophthalmologists need to have an understanding of the myriad factors that contribute to DED in order to approach it in a sensible way,” said Kathryn Colby, MD, PhD, at the University of Chicago.
Grappling with complexity. The vast majority of DED patients have evaporative dry eye; a minority have aqueous deficiency; and a much smaller number have mucin deficiency, neuropathic pain, or other subtypes. Subtyping can help a clinician decide what to address first, but subtypes often overlap, which can create confusion.
“Dry eye is an extremely heterogeneous entity,” said Bennie H. Jeng, MD, at the University of Maryland School of Medicine in Baltimore. “Even within a subclass, you can’t necessarily turn to a single algorithm of how to treat it. You can have an idea of an algorithm but must realize that not every patient is going to respond the same way. You have to think creatively about different treatment pathways.”
What DEWS II recommends. DEWS II’s proposed process starts with triage questions and risk factor analysis. The clinician should take a thorough medical history, covering the patient’s ocular history (surgical history, contact lens use, etc.), systemic medications, ocular medications, allergies, chief complaints and current symptoms, and prior and current treatments for DED.
DEWS II then suggests that clinicians move on to basic diagnostic testing followed by subtype classification tests (understanding that a broad range of DED encompasses more than 1 subtype). Next, the clinician should start appropriate treatment, escalating the approach as needed (see “A Staged Approach to Treatment”).
Pearls for thinking it through. “The first thing I want to know is whether we’re talking about symptomatic or asymptomatic disease,” said Dr. Galor. Is this something you’re seeing on staining but the patient has no symptoms, or is the patient complaining of either painful ocular surface symptoms or visual disturbances?
“Painful ocular surface symptoms are caused by nerve stimulation, and I want to know what is causing nerve activation,” Dr. Galor said. “Visual symptoms, like fluctuating vision, are most often an issue of tear film parameters. Sometimes you have both, but the first thing is figuring out what exactly the patient is complaining of.”
Verify the diagnosis. The first thing Dr. Jeng does when seeing a patient is to verify that the patient actually has DED, given the frequency of misdiagnosis. “While I’m talking to patients, without drawing attention to it, I study their blink rate and look at the positioning of their eyelids. These are important in deciphering whether dry eye is the primary problem or maybe an exposure problem,” said Dr. Jeng.
For example, perhaps patients don’t blink enough because they have early Parkinson disease, or they don’t close their eyes all the way because their lower lid is sagging. For these patients, the problem is exposure, not tear deficiency or meibomian gland dysfunction. (Dr. Jeng also tries to simulate nocturnal lagophthalmus to test for this exposure risk.) To ferret out cases of tear deficiency, he asks patients whether they are able to cry.
Investigate the pain. Most of Dr. Galor’s patients have painful ocular surface symptoms, such as sensations of dryness, burning, and aching. In these patients, she tries to determine what underlies their nerve activation. Is it nociceptive pain (something on the ocular surface that is activating the nerve) or neuropathic pain (the nerves themselves are dysfunctional)?
When it comes to nociceptive pain, the most common cause is an unstable tear film. One subtype of DED is aqueous deficiency, which often occurs in patients with Sjögren syndrome or other collagen vascular diseases, such as rheumatoid arthritis. These patients don’t make enough tears, which leaves their nerves exposed and irritated, but that’s a small subset of patients with painful symptoms. Most do not have a tear production problem, so what else is going on?
Inflammation may be the culprit, she noted. “People have inflammation as an endpoint for many different pathways: allergic, toxic, tear film abnormalities, to name a few,” said Dr. Galor. Once she identifies inflammation as a component of the painful symptoms, she knows to treat it.
Consider the anatomy. You also have to think about anatomy, advised Dr. Galor. “Lumps, bumps, laxity, and redundancy of the eyelids and/or conjunctivae affect ocular surface dynamics, which in turn affect sensation and homeostasis. These are all comorbid and may be the primary contributor to painful symptoms.”
Anatomy is something clinicians can usually fix, although some conditions can be addressed more easily than others. As Dr. Galor noted, “The reason it gets messy is that, any time you have an anatomic abnormality, it affects the tear film. So, which came first, the chicken or the egg?”
 |
|
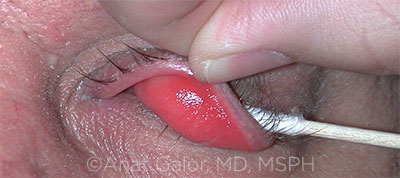
EYELID LAXITY. The presence of eyelid laxity has been associated with abnormal tear parameters. In this image, note the upper conjunctival papillary changes.
|
Testing: Where to Start
Ocular surface staining. “The most important test for me is ocular surface staining,” said Dr. Jeng, “not just fluorescein staining of the cornea but also lissamine green staining of the conjunctiva.” Dry eye gives a specific pattern of staining, usually the inferior cornea and a little bit on the limbus. If it’s widespread and severe, you’ll get diffuse staining and filaments, but for run-of-the-mill dry eye, you’ll get inferior staining.
“If you see diffuse staining or superior staining, these are findings that signal other things, such as superior limbic keratoconjunctivitis (SLK),” said Dr. Jeng. Patients with SLK have complaints similar to dry eye patients, for example foreign body sensation and dryness, but the staining pattern distinguishes the two. (With SLK, there is superior corneal and superior conjunctival staining.) As for diffuse staining, it can be seen with other corneal diagnoses—for example, after a chemical injury.
Patients who use eyedrops to treat what they think is dry eye can get toxicity from the preservatives (particularly benzalkonium chloride) or the drops themselves. Specific staining patterns, such as staining along the inferior palpebral conjunctiva that leads to the puncta, can alert you to this.
Additional tests. Besides staining, Dr. Jeng measures tear-film break-up time (TBUT). It’s also important to look at the meniscus and the meibum that’s expressed from the meibomian glands, he said.
What about point-of-care testing? Dr. Jeng does not use ancillary testing. “Though [point-of-care tests are] potentially useful as an adjunct or confirmation, for me, none of the new tests are as good as the basics we already have for actually making the diagnosis,” he said.
Need for bigger picture. “Everyone wants 1 test that tells us ‘yes, you have dry eye’ or ‘no, you don’t,’ and many companies market their tests that way, but dry eye includes lots of different conditions, so it’s unrealistic to think that a single test can tell us ‘yes’ or ‘no,’” said Dr. Galor.
She likens the challenge with point-of-care testing to HbA1c versus blood glucose testing. “Whereas blood glucose testing measures a patient’s glucose level at a single point of time, HbA1c gives a 3-month picture. We need something like that—an intelligent dry eye test that measures stability on a bigger scale—not just a snapshot for that moment in time, which may not accurately represent the condition.” (See “Point-of-Care Tests: What Are You Measuring?”)
Useful for subtyping. Despite these limitations, some of the new point-of-care tests can be useful for subtyping dry eye, according to Dr. Galor. There are tests to measure osmolarity (such as TearLab’s Osmolarity System) and inflammatory markers (such as Quidel’s InflammaDry). If Dr. Galor thinks there could be meibomian gland atrophy in a young patient, she might use LipiView (TearScience), which highlights the anatomy.
“Unfortunately, we’re measuring all these static markers and hoping they’ll give us a picture of what’s going on. But, in truth, everything—anatomy, eyelid movement, the tear film, tear production, inflammation—is contributing to the problem,” said Dr. Galor. “It’s all dynamic, and we’re hoping that the metrics we use capture it.”
 |
|
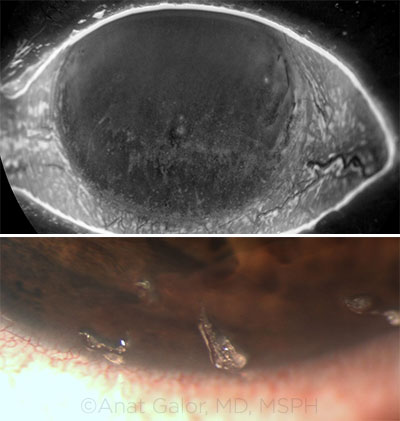
TEARS. (Top) Corneal staining in the setting of tear film dysfunction. (Bottom) Filaments in a patient with aqueous tear deficiency.
|
Thoughts on Treatment
Although the DEWS II treatment subcommittee developed stepwise management and treatment recommendations (see “A Staged Approach to Treatment”), they also warned that a straightforward algorithm for treating DED is not possible, as “it is a complex condition that varies, both in severity and in character, from patient to patient.”
Trial and error. “There’s a real art to dry eye management. While much of that comes from experience, it’s important to think of dry eye as a multitreatment disease,” said Dr. Colby.
As an example, Dr. Colby noted that it’s not uncommon for a general ophthalmologist or an optometrist to see someone with dry eye and decide to put plugs in. “But if a patient has evaporative dry eye with inflammatory debris in their tears and you plug their tear drainage system, you’re going to make their symptoms worse, not better. If someone came in very inflamed,” said Dr. Colby, “I’d probably put them on a little steroid and some doxycycline, and then, the next time they came in, put the plugs in.”
What about omega-3 supplements? In recent years, a growing number of ophthalmologists have recommended dietary supplementation with omega-3 fatty acids as an adjunct treatment to help suppress DED-related inflammation. But results from a study funded by the National Institutes of Health may upend that strategy.
In May, the Dry Eye Assessment and Management Study Research Group published what Dr. Jacobs called “jaw-dropping results.” The study, published in The New England Journal of Medicine, found no difference between the supplements and placebo.3
According to Dr. Jacobs, the study’s results illustrate the problem of approaching dry eye as a monolithic syndrome. “If you do a high-power study with a lot of patients and enroll them by dry eye criteria, you may not be able to get a treatment effect, because it’s such a mixture of subtypes,” she said.
While Dr. Colby agreed that a heterogeneous trial population always reduces the signal, she also noted that both the omega-3 fatty acid group and the control group, which was given olive oil supplements, improved. Dr. Colby’s takeaway wasn’t that omega-3 supplementation is not helpful but rather that olive oil may be helpful, too.
What about LipiFlow? A new treatment on the market is the LipiFlow thermal pulsation system (TearScience), which targets meibomian gland dysfunction. The in-office procedure applies heat to the eyelids to melt waxy deposits in the meibomian glands. It simultaneously applies pulsed pressure to the eyelid to open and express the contents of the glands.
While some clinicians are fans of the LipiFlow system, it isn’t used by the experts interviewed for this article. Old-school warm compresses and lid scrubs are just as effective and far less expensive, they said. Dr. Jeng did note that some of his colleagues like the technology because they say it can show patients how they’re improving.
Caution advised. Dr. Jacobs advised a mix of cautious optimism and skepticism whenever new DED diagnostics and therapeutics hit the market. “The problem is that there can be discrepancies between what patients come in with, what doctors tell them they have, and what industry has to sell clinicians and patients,” she said. “These are not always well aligned, even if they all fall under the umbrella of dry eye.”
Dr. Colby added, “The pharmaceutical approach to dry eye is to find a marker and then try to control it, but it’s analogous to taking a pot of spaghetti and throwing it against the wall, hoping something sticks. Until we can say, ‘Here’s the pathophysiology of dry eye; let’s design an intervention that treats the cause,’ we’re not going to make significant progress.”
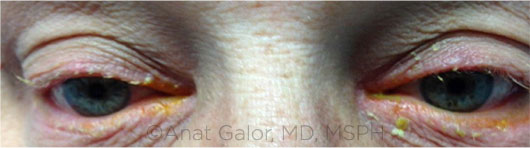 |
|
BLEPHARITIS. Anterior blepharitis (shown here) may have a bacterial etiology
|
Additional Notes
A note on patient communication. Patient education is critical in managing expectations. Given his position in the chain of referral, Dr. Jeng is often the third or fourth physician his patients have seen. “Communication is paramount because patients don’t understand why they’re back in the office for the 11th time and still haven’t been cured. You have to explain that it’s not a one-and-done thing. It’s a long haul.”
Dr. Jeng cites blepharitis—1 cause of dry eye—as an example. He always tells blepharitis patients the first time he sees them that there is no cure for them. “I assure them that we will tryto make them more comfortable and optimize their condition, but I explain that blepharitis is a chronic skin condition,” he said. He then outlines what he’s going to do, emphasizing that there will be some trial and error.
A note on cataract surgery. It is essential to diagnose DED preoperatively and to treat the ocular surface—even in asymptomatic patients—before taking preoperative measurements for cataract surgery. “If the ocular surface isn’t in a homeostatic state, the day of the measurement may be different from the day of the surgery, leading to poor results,” Dr. Jacobs said. Do not rush into surgery, she and the other experts advised, and use caution with premium intraocular lenses in patients with a history of dry eye.
A note on referral. It is appropriate to refer a patient to a cornea or dry eye specialist when corneal epithelium breakdown is a concern despite a lack of symptoms and despite several attempted treatments, Dr. Colby said. She added, “If you’re thinking [the breakdown] could be related to Sjögren syndrome or another systemic disease, or if you’re not able to get your patient comfortable despite trying several interventions, that might be a time to refer as well.”
___________________________
1 Market Scope. 2016 Dry Eye Products Report: A Global Market Analysis for 2015 to 2021. St Louis: Market Scope; 2016.
2 Craig JP et al. Ocul Surf. 2017;15(3):802-812.
3 Asbell PA et al, for the Dry Eye Assessment and Management Study Research Group. N Engl J Med. 2018;378(18):1681-1690.
A Staged Approach to Treatment
The following information is adapted from the DEWS II Management and Treatment Report.1 In general, treatment begins with low-risk strategies and progresses as needed.
Stage 1. This includes modification of the patient’s environment; potential dietary modifications; identification and potential modification or elimination of offending systemic and topical medications; ocular lubricants; and lid hygiene and warm compresses.
Stage 2. This includes nonpreserved ocular lubricants; tear conservation, including punctal occlusion; overnight treatments, such as an ointment; in-office expression of the meibomian glands; and prescription drugs, including topical anti-inflammatory agents.
Stage 3. This includes oral secretagogues; serum eyedrops; and contact lenses.
Stage 4. This includes topical corticosteroids; amniotic membrane grafts; and surgical punctal occlusion.
Caution. Per the report, “It is acknowledged that the significant heterogeneity that exists in the DED patient population precludes an overly formulaic approach and it would be anticipated that these recommendations would be adapted, by eye care practitioners, to best suit individual patients.”
___________________________
1 Jones L et al. Ocul Surf. 2017;15(3):575-628.
|
Point-of-Care Tests: What Are You Measuring?
Here’s a brief overview of which measures are targeted by several point-of-care tests.
Measuring lipid thickness. Lipid layer thickness is marketed as a measure to help decide whether to proceed with LipiFlow (designed to treat meibomian gland dysfunction), with the idea that a thin central lipid thickness is “bad” and a thick layer is “good.” But some clinicians have found that central lipid thickness is of limited value in diagnosis or predicting therapy.
“The reason is that there are people with a lipid layer of 30 nm that is uniform throughout the cornea and remains stable over time; then there are people with a central lipid layer of 70 nm that is unstable, with values of 70 nm centrally and 30 nm peripherally that dissipate quickly,” said Dr. Galor. “If you can’t capture the dynamics of the system, then the measure isn’t as helpful.”
Measuring tear osmolarity. This is “a very useful measure, but what you want to know is stability over time, given that the hallmark of dry eye is instability,” Dr. Galor said. Unfortunately, each test chip costs $15 and you can’t check osmolarity 3 times in a row in a single visit. One measure of tear osmolarity isn’t as useful—not because osmolarity is a bad metric, but because you don’t get a good enough picture of homeostasis. Dr. Colbysaid that she stopped using the tear osmolarity test “because it cost us more to do [it] than we got reimbursed, and we weren’t sure exactly how this data changed our management.”
Measuring inflammation. InflammaDry measures the inflammatory marker MMP-9. While the test is qualitative (>40 mg/mL is positive), Dr. Galor estimates the degree of inflammation by the intensity of the pink line in the test’s results window and grades it as faint (minimal), light pink (mild), pink (moderate), or fuchsia (severe inflammation). She considers a pink or fuchsia line an indication that inflammation is an important contributor to the disease process.
Dr. Galor acknowledged that some of her colleagues dislike the test “because it’s qualitative and because there are other pathways of inflammation besides MMP-9. It’s also unclear whether inflammation on the ocular surface is representative of what is happening elsewhere in the lacrimal functional unit, such as whether it represents inflammation in the lacrimal gland,” she explained, “but at least it’s a start in the right direction.”
|
Meet the Experts
Kathryn Colby, MD, PhD Louis Block professor and chair of ophthalmology at the University of Chicago. Relevant financial disclosures: None.
Anat Galor, MD, MSPH Associate professor of clinical ophthalmology and visual science at Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: Allergan: C; Novaliq: C; Shire: C.
Deborah S. Jacobs, MD Faculty at Massachusetts Eye & Ear’s Cornea Service and associate professor of ophthalmology at Harvard in Boston. Relevant financial disclosures: None.
Bennie H. Jeng, MD Professor and chair of ophthalmology and visual sciences at the University of Maryland in Baltimore. Relevant financial disclosures: None
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Kathryn Colby, MD, PhD WL Gore: C.
Anat Galor, MD, MSPH Allergan: C; Novaliq: C; Shire: C.
Deborah S. Jacobs, MD BostonSight: E; TECLens: C.
Bennie H. Jeng, MD Aerie: C; Aquinox: C; EyeGate: C,O; Kedrion: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|