By Alex G. Mcgaughy, BS, and Preeya K. Gupta, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Human amniotic membrane (AM) has been shown to provide a substantial benefit in treating certain types of conjunctival and corneal disease. While AM has traditionally been transplanted using sutures or fibrin glue in an operating room, the in-office application of sutureless AM is emerging as a less invasive alternative with promising clinical outcomes. In-office availability of AM allows clinicians to treat a variety of ocular surface conditions rapidly and effectively.
What Is Amniotic Membrane?
Amniotic membrane is an avascular fetal membrane that lies deep to the chorion and is harvested in a sterile environment from placental tissue obtained during elective cesarean sections. Donors are screened for transmissible diseases, and the AM is further treated with broad-spectrum antibiotics immediately after collection.
AM is made up of three layers: epithelium, basement membrane, and stroma. Several types of collagen make up the basement membrane, including type VII collagen, which is also present in the conjunctival and corneal basement membranes. The stroma of AM can be subdivided further into a compact layer, a fibroblast layer, and an outer spongy layer.1
Beneficial properties of AM. Several characteristics contribute to the efficacy of AM in treating ocular surface problems. First, AM acts as a physical barrier to protect conjunctival and corneal epithelium as it heals, and it reduces pain caused by friction of the eyelids over the surface. In addition, the AM basement membrane promotes epithelial growth through cell migration, adhesion, and differentiation, while also inhibiting cell death.2,3 Furthermore, the stroma of AM, which contains fetal hyaluronic acid, inhibits fibroblast growth and reduces inflammation through decreased expression of cytokines.
Research has also shown AM to block angiogenesis and have antimicrobial properties.1 The mechanism of the antimicrobial effect is controversial, but AM may decrease the risk of infection either through inherent chemical properties or through its function as a physical barrier.
AM is universally tolerated due to its lack of histocompatibility antigens HLA-A, B, or DR; it can be implanted without concern for rejection.
Types of Amniotic Membrane
Currently, two main types of AM are commercially available for in-office use: cryopreserved and dehydrated. Both types come in a variety of tissue thicknesses and sizes, depending on clinical needs.
Cryopreserved AM. Cryopreservation of AM involves slow freezing at –80°C using DMEM/glycerol preservation media to allow for slow-rate freezing without ice formation. This preservation technique retains the extracellular matrix components, such as heavy-chain hyaluronic acids, growth factors, fibronectin, and collagen, all of which promote anti-inflammatory effects and healing. The tissue is stored in a –80°C freezer and brought to room temperature when needed for use.
ProKera (BioTissue) is a cryopreserved form of AM in which the membrane is secured around a polycarbonate ring or an elastomeric band. It requires no assembly and is inserted into the eye in a manner similar to contact lens placement. This form of AM has been cleared by the FDA as a class II medical device, and product claims approved by the FDA include protective, wound healing, and antiinflammatory effects.
Dehydrated AM. Dehydrated AM is preserved using vacuum with low temperature heat to retain devitalized cellular components. FDA-approved claims for this type of AM are limited to wound coverage. Unlike cryopreserved tissue, dehydrated AM is kept at room temperature, but it must be rehydrated for clinical use.
AmbioDisk (IOP Ophthalmics) is a dehydrated AM commercially available for in-office use; it is applied directly to the ocular surface and covered with an overlying bandage contact lens.
Other manufacturers of this type of tissue include BioDOptix and Seed Biotech.
 |
|
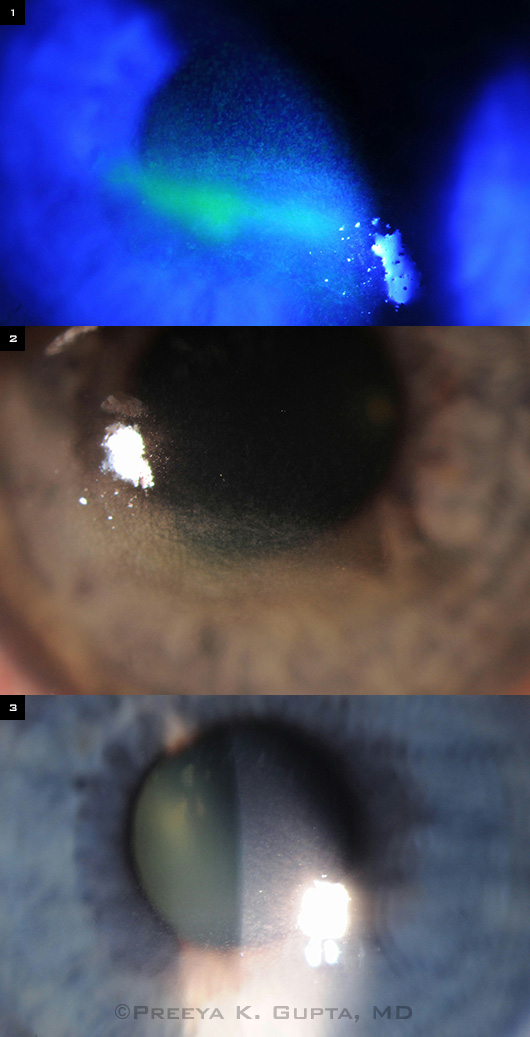
CASE STUDY. (1,2) This 32-year-old woman had a long history of diabetes, dry eye, and exposure keratopathy (worse in the right eye) due to incomplete blinking and lagophthalmos after ptosis repair. She presented with blurry vision that was recalcitrant to topical lubricants, oral doxycycline, bandage contact lens placement, and punctal plugs. Her BCVA was 20/100 OD. ProKera Slim was inserted for seven days. (3) After the device was removed, BCVA had improved to 20/25, and corneal staining was resolved.
|
How to Apply AM in the Office
ProKera
- Remove package from freezer and allow device to come to room temperature (typically 5 minutes when left on exam room table).
- Open pouch carefully and drain preservation fluid from package.
- Rinse the ring/membrane with sterile saline to remove glycerin.
- Place topical proparacaine on the ocular surface.
- Insert superior ring edge under the upper eyelid while patient is looking down, and then have patient look up while you place remainder of ring onto the eye; the membrane is supplied in the correct orientation such that the stromal side will be in contact with the ocular surface.
AmbioDisk
- Remove the disk carefully from inner package—the dehydrated tissue is very thin and light in weight.
- Place topical proparacaine on the ocular surface.
- Place the AM disk onto the ocular surface. Orient the graft with the “IOP” watermark facing the physician and reading correctly to ensure that the stromal side is in contact with the eye.
- Smooth the graft onto the ocular surface.
- Place a few drops of sterile saline to rehydrate the membrane.
- Place a bandage contact lens to cover the membrane and corneal surface.
Clinical Applications
Sutureless AMs are valuable in the office setting to promote healing of ocular surface conditions that have the potential to create corneal scarring or are not responding to treatment. These include various types of keratitis, corneal ulcers, neurotrophic keratopathy, and chemical burns.4
Keratitis. There are several causes of keratitis and keratoconjunctivitis, which can be broken down into infectious and noninfectious etiologies. Infectious etiologies include all types of microbial involvement such as bacterial, viral, fungal, and parasitic. Infectious keratitis should always be treated with the appropriate antimicrobial medication before use of AM.
Noninfectious causes of keratitis include dry eye–related inflammation, contact lens overwear, and autoimmune diseases.
Neurotrophic keratitis is a serious condition that can result from corneal nerve damage, most frequently caused by trauma or herpetic infection. Sensory nerve impairment leads to degeneration of the corneal epithelium secondary to dryness and mechanical injury. This type of keratitis can be difficult to manage with traditional topical therapies.
Early intervention. In contrast to prior treatment algorithms for ocular surface diseases that introduced AM late in the protocol, early intervention with AM is important. In-office use of this tissue as an adjunct to other forms of treatment can facilitate rapid wound healing, avoid complications such as corneal melt or secondary infection, resolve punctate staining more rapidly, and reduce scar formation—thus improving final visual outcome.4
Topical medications can be used concurrently, as medication penetration has not been found to be an issue.
Patient Experience and Side Effects
Patients generally tolerate AM very well. Temporary blurring of vision is caused by the AM covering the cornea.5 In our clinical experience, patients sometimes report mild discomfort with the ProKera, presumably due to its polycarbonate ring.
The AM typically dissolves over one week, though it can last longer depending upon the clinical condition. With the ProKera device, the ring is removed by the physician after the membrane has dissolved.
Conclusion
The convenience, safety, and effectiveness of sutureless AM are helping to produce excellent clinical outcomes and revolutionize the treatment of a variety of ocular surface diseases. In-office AM is easy to use and could become a part of treatment algorithms earlier in the disease course to improve patient outcomes.
___________________________
1 Malhotra C, Jain AK. World J Transplant. 2014;4(2):111-121.
2 Meller D, Tseng SC. Invest Ophthalmol Vis Sci. 1999;40(5):878-886.
3 Boudreau N et al. Proc Natl Acad Sci USA. 1996;93(8):3509-3513.
4 Suri K et al. Eye Contact Lens. 2013;39(5):341-347.
5 Ijiri S et al. Am J Ophthalmol. 2007;144(6):938-942.
___________________________
Mr. McGaughy is a medical student at Medical University of South Carolina, in Charleston; he reports no related financial interests. Dr. Gupta is an assistant professor of ophthalmology at the Duke University Eye Center, and is clinical director of the Duke Eye Center at Page Road, Durham, N.C. She reports she is a consultant to BioTissue.