Download PDF
Canadian researchers have shown that lymphatic vessels are implicated in corneal transplant graft failure with neovascularization.1 “Our study proves for the first time the presence of lymphatics in failed vascular corneal grafts and [shows] that they are distinct from blood vessels,” said Neeru Gupta, MD, PhD, MBA, at the University of Toronto. “This work highlights the role of lymphatics in corneal transplant failure and points to a need to develop novel treatments that target lymphatic vessels to help manage the failing graft,” she added.
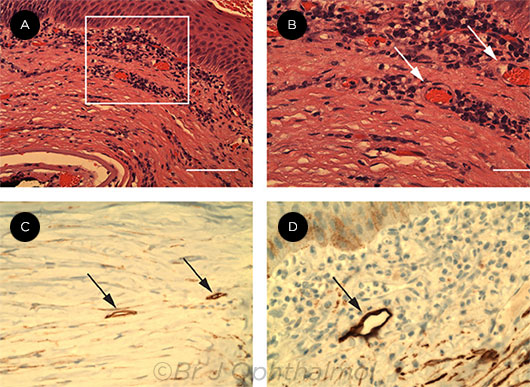 |
FAILED GRAFTS. These images show neovascularization and suspected lymphatics within failed corneal grafts. Blood vessels shown at 20× magnification (rectangular area, A) and at 40× magnification (arrows, B). Immunoperoxidase images show podoplanin-antibody staining lymphatics (arrows, C and D). Michael Adam Diamond, Sze Wah Samuel Chan, Xun Zhou, Yelena Glinka, Eileen Girard, Yeni Yucel, Neeru Gupta. Br J Ophthalmol. 2019;103(3):421-427. Reprinted under Creative Commons Attribution Noncommercial (CC BY-NC 4.0) license. Image caption adapted from the original (Fig. 1, page 423).
|
Tissue collection. For this study, failed corneal transplant cases were selected from the Toronto Ophthalmic Pathology database. Of 273 cases, 39 contained documented neovascularization. Of these, nine cases (six men, three women) also contained suspected lymphatics. The researchers then obtained conjunctival tissue from six patients (three men, three women) with healthy corneas. These control cases were acquired from the Human Eye Biobank for Research, also located in Toronto.
Methods. The researchers selected the nine failed grafts based on results of immunohistochemistry (IHC), immunofluorescence (IF), H&E staining, and immunoperoxidase staining for CD31, a blood vessel marker. In addition, for two of these cases, they used fluorescence in situ hybridization (FISH) to detect lymphatic mRNAs, including podoplanin. All IF and FISH samples were compared with positive and negative controls and visualized by confocal microscopy.
Results. Podoplanin-immunoreactive lymphatics were detected in all nine failed grafts by IHC; of these, seven also were positive by IF. Moreover, two of the cases were positive for at least two lymphatic markers simultaneously.
H&E stained sections of failed grafts showed mononuclear inflammatory cells at both low and high power, and neovascularization was confirmed in every case of corneal graft failure by detection of CD31-positive profiles. Varying lymphatic sizes and morphologies were seen both among separate cases and within a single case, and myriad unique lymphatic morphologies were seen.
Next steps. The researchers emphasized that their findings stress the importance of developing new tools, therapies, and imaging modalities to bring about improvements in graft survival.
—Arthur Stone
___________________________
1 Diamond MA et al. Br J Ophthalmol. 2019;103(3):421-427.
___________________________
Relevant financial disclosures—Dr. Gupta: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Colby WL Gore, Inc.: C.
Dr. Gupta None
Dr. Moore None
Dr. Hesse None
Dr. Rosen Astellas: C; Bayer: C; Boehringer Ingelheim: C; Diopsys: C; Genentech/Roche: C; Guardion Health: O; Nano-Retina: C; OD-OS: C; Opticology: O; Optovue: C; Regeneron: C; Teva: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review