Download PDF
This month, News in Review highlights selected papers from the original papers sessions at AAO 2019. Each was chosen by the session chairs because it presents important news or illustrates a trend in the field. Only four subspecialties are included here; papers sessions will also be held in six other fields. For more information, see the Mobile Meeting Guide.
British researchers set out to evaluate corneal neurotization in patients with neurotrophic keratopathy. They found that the surgical procedure restores trophic and sensory functions of neurotrophic corneas.
“Restoration of corneal sensation contributes to improved corneal function and structural health,” said Samer Hamada, MD, at Queen Victoria Hospital in East Grinstead, England, and Eye Clinic London. “This helps prevent complications of neurotrophic keratopathy.”
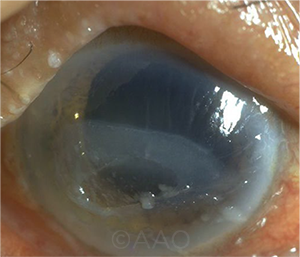 |
NOVEL TX. Corneal neurotization is a new option for restoring sensation and improving vision in patients with neurotrophic keratopathy.
|
Patient selection. For this prospective study, 11 patients with neurotrophic keratopathy of various degrees underwent sural nerve transplantation surgery between February 2016 and April 2018 at Queen Victoria Hospital. A multidisciplinary team of cornea, oculoplastic, and plastic surgeons was involved.
The patients selected for this procedure had significant ocular morbidity secondary to irreversible neurotrophic keratopathy, and they had already undergone failed conventional medical or surgical treatments.
Methods. Outcome measures included visual acuity (VA) and evaluation of ocular surface (OS) staining, tear production, tear film breakup time (TFBT), osmolarity, and corneal sensation. Structural outcomes were assessed for changes in corneal nerve density and morphology by in vivo confocal microscopy.
Both functional and structural outcomes were measured preoperatively and postoperatively at the early (1-3 months), intermediate (3-6 months), and late (9 or more months) stages. Objective evidence for worsening keratopathy included reduced VA, TFBT, tear meniscus height, tear film quality and osmolarity, corneal thickness, and increased corneal and conjunctival staining.
Results. At last follow-up, VA had stabilized and improved in 10 patients. In addition, OS staining improved in 10 patients, tear quantity and quality improved in nine, tear film osmolarity was reduced in eight, and corneal sensation improved in seven. No complications were recorded intra- or postoperatively, and three patients had general improvement in sub-basal corneal nerve length and density. No patients had ulcers after the procedure.
Patient feedback. Six patients filled out questionnaires before and after surgery. One reported poor post-op vision; the remainder graded it to be fair to good. Reading improved in four patients from being extremely or moderately difficult preoperatively to experiencing no or mild difficulty following the procedure. The other two patients reported no changes.
—Arthur Stone
|
Functional and Structural Changes Following Corneal Neurotization in the Management of Neurotrophic Keratopathy. Presented during the cornea and external disease original papers session. When: Monday, Oct. 14, 2:00-4:30 p.m. Where: South 10. Access: Free.
|
___________________________
Relevant financial disclosures—Dr. Hamada: None
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Hamada None.
Dr. Lin None.
Ms. Shoshany None.
Dr. Swaminathan None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review