Download PDF
Presumed ocular histoplasmosis syndrome (POHS) is an inflammatory, multifocal chorioretinal disorder. It was initially called ocular histoplasmosis syndrome because of its prevalence in areas with endemic histoplasmosis infection. However, according to more recent studies, this relationship has not been definitively proved; thus, “presumed” was added to the name.1 Although the disease was first described 80 years ago, its etiology, pathogenesis, and treatment remain elusive and controversial.
POHS is diagnosed based on clinical findings characterized by a triad of peri- papillary atrophy, chorioretinal macular and midperipheral scars, and choroidal neovascularization (CNV). In contrast to the fundus, the anterior segment and vitreous cavity are spared from inflammation.2 The presentation can vary from asymptomatic to central vision loss. It is one of the main causes of severe irreversible vision loss in middle-aged adults who live in endemic regions.3
Epidemiology
Histoplasmosis, which is detected by a histoplasmin skin test, is the leading cause of endemic mycosis in the world.2 In the United States, POHS is most commonly found around the Mississippi and Ohio River valleys, where histoplasmosis is endemic.4 The incidence of histoplasmosis in the endemic areas of the United States is between 200,000 and 500,000 cases per year.5 The disease is most common among white adults between 20 and 50 years old, with no gender predilection.2
In countries other than the United States, the prevalence of POHS is not related to histoplasmosis dispersion.
 |
|
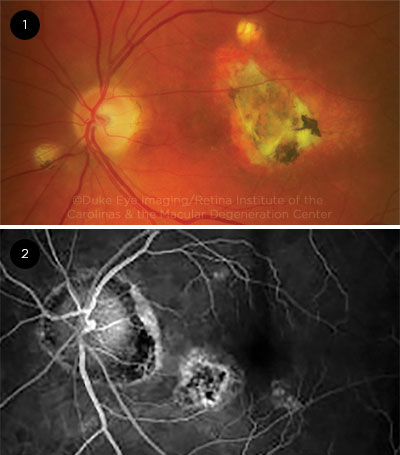
IMAGES. (1) Fundus photograph shows typical punched-out lesions, peripapillary atrophy, and choroidal neovascularization with subretinal fluid and blood in the macula. (2) FA shows punched-out lesion and peripapillary atrophy.
|
Etiology and Risk Factors
There is ongoing debate about the etiology of POHS. The strongest hypothesis is infection by Histoplasma capsulatum (Hc). The principal route of infection is inhalation of infectious spores or conidia form of fungus; Hc is not transmitted from person to person or from animal to person. Sources of infection include surface soil fertilized by bat or bird droppings, old buildings, bat caves, aviaries, and dead trees.6
Evidence for this hypothesis includes the high prevalence of POHS in histoplasmosis-endemic areas, high rate of positive histoplasmin skin test in patients with POHS, and signs of POHS after intra-arterial inoculation of Hc in animal studies. However, POHS is also found in nonendemic countries like the Netherlands, India, and the United Kingdom.
Many studies show that genetic factors may play a role in this disease. Certain human leukocyte antigen sub-types (DR1, DQ6, DR15, DRw2, and B7) can be predisposing factors for POHS.2,4,7 Another hypothesis is that an infection by Epstein-Barr virus, by another systemic mycosis, or by any organism that produces an antigen similar to that of Hc, can trigger the immune system by means of cross-reactivity.2
Risk factors for developing CNV in patients with POHS include smoking, increasing age, and low levels of education. Smoking is the leading risk factor: The probability of developing CNV is three times higher in smokers than in nonsmokers.3
Fellow eye prognosis depends on its baseline condition. In fellow eyes without any signs of neovascular maculopathy, 9% will progress to CNV, with an annual incidence of 1.8%. The presence of maculopathy or choroidal midperipheral scars (often called histo spots) increases the probability of progression.2
Pathophysiology
The precise pathophysiology of the disease is unclear, but the hypothesis that most scientists support is that the Hc spores enter the lungs of a patient by inhalation. They are then phagocytosed by alveolar macrophages and replicate. Eventually, they move to the blood, cause fungemia, and finally seed in the choroidal layer of the eye. This seeding causes inflammation by lymphocytic infiltration, damages Bruch membrane and the retinal pigment epithelium (RPE), and leads to formation of chorioretinal scars. With repeated exposure to the Hc spores and continuation of this cycle, more lesions may develop, and the chance of CNV may increase.2
Clinical Presentation
POHS is not associated with vitritis or anterior chamber inflammation. The main target of the disease is the fundus. Therefore, POHS diagnosis is defined clinically by the following triad of signs:
- Peripapillary atrophy (PPA)
- Choroidal midperipheral scars (histo spots), which appear as “punched-out” lesions, along with similar macular scars
- CNV or subsequent disciform scarring
In general, the POHS diagnosis is made when at least two of these three criteria are met in one or both eyes.
In the early phase, POHS is asymptomatic and is characterized by deep, distinct, round, creamy white lesions. It should be noted that PPA and histo spots do not cause visual symptoms.2 Enlarging macular lesions that change in color from yellow to gray-green signify development of CNV.2,4
Later-stage POHS may affect vision with the development of CNV, retinal edema, and intraretinal hemorrhage. Symptoms include metamorphopsia, blurred central vision, central or paracentral scotoma, and painless vision loss. Untreated CNV can lead to disciform lesions and, potentially, vision loss.2,3
Diagnostic Approach
There are no specific laboratory tests for diagnosing POHS. Diagnosis is clinical, based on a dilated funduscopic examination and the presence of at least two criteria of the classic triad of the disease.
Imaging. The following modalities may be helpful in diagnosing and monitoring POHS.
Fluorescein angiography. The appearance of CNV due to POHS is the same as that caused by other retinal conditions. Window defect pattern of hyperfluorescence and progressive scleral staining are seen with PPA and chorioretinal scars.
Indocyanine green angiography. ICGA can be used to detect sub-RPE, or so-called occult, CNV lesions. Disorganization of the choriocapillaris manifests as increased hyperfluorescence on early-phase ICGA.
Spectral-domain optical coherence tomography. SD-OCT is used to observe the disease course. When PPA and/or histo spots are present, the normal hyperreflective bands appear disorganized. Histo spots also appear as focal areas of outer retinal atrophy.
Fundus autofluorescence. FAF is a reliable method for detecting small, nonpigmented macular chorioretinal scars, which are distinguished by their round, hypoautofluorescent appearance.2
Differential. Other conditions that can have ocular signs and symptoms similar to POHS include the following:
- Diseases that induce CNV, such as age-related macular degeneration, idiopathic CNV, and myopia
- White dot syndromes, including multifocal chorioretinitis with panuveitis, punctate inner choroidopathy, and multiple evanescent white dot syndrome
- Systemic disorders such as sarcoidosis and toxoplasmosis.2
To distinguish POHS from other conditions, look for the triad of PPA, histo spots, and maculopathy, all in the absence of vitritis, plus a medical history with potential risk factors (e.g., history of living in histoplasmosis-endemic region).
Management
POHS is not treated unless—and often remains undiagnosed until—CNV develops. The goal of POHS management is to detect and treat CNV as early as possible to prevent further complications and visual loss. If on funduscopy, POHS lesions such as histo spots or PPA are observed but no CNV is seen (asymptomatic POHS), it is recommended that patients have regular dilated funduscopic examinations and check their vision by Amsler grid daily.2
Anti-VEGF therapy. Anti-VEGF is typically the first-line treatment for CNV associated with POHS. It has been shown to be effective and have fewer side effects compared with surgery or laser photocoagulation.2,3,8
Photodynamic therapy. PDT also shows promising results, especially in treating subfoveal and juxtafoveal CNV secondary to POHS. It can be used as an adjunct to anti-VEGF in the refractory cases that respond poorly to anti-VEGF therapy.2,3
Laser. Laser photocoagulation is generally limited to extrafoveal CNV as well as some cases of juxtafoveal CNV.
Other considerations. Although intravitreal and systemic corticosteroids have been shown to be slightly beneficial, they are used infrequently because they are less effective and have more adverse effects than other treatments.2
Antifungal therapy has not been demonstrated to be effective and, therefore, has been abandoned as a therapeutic modality for POHS.2-4
___________________________
1 Behrens-Baumann W et al. Klinische Monatsblatter fur Augenheilkunde. 1988;192(4):348-353.
2 Diaz RI et al. Surv Ophthalmol. 2015;60(4):279-295.
3 Thuruthumaly C et al. Curr Opin Ophthalmol. 2014;25(6):508-512.
4 Oliver A et al. Curr Opin Ophthalmol. 2005;16(3):160-165.
5 Deepe GS. Curr Opin Microbiol. 2000;3(4):359-362.
6 Elfervig LS, Elfervig JL. Insight. 1998;23(4):130-132.
7 Dabil H et al. Hum Immunol. 2003;64(10):960-964.
8 Toussaint BW et al., for the HANDLE study. Retina. 2018;38(4):755-763.
___________________________
Dr. Akhavanrezayat is an ophthalmology research fellow and Dr. Samiy and Dr. Farr are retina specialists; all are at the Retina Institute of the Carolinas & the Macular Degeneration Center in Charlotte, N.C. Financial disclosures: None.