By Rohan Bajaj, BS, Gislin Dagnelie, PhD, James T. Handa, MD, and Adrienne W. Scott, MD
Edited By: Ingrid U. Scott, MD, MPH, and Bennie H. Jeng, MD
Download PDF
One in 3,500 people in the United States and Europe is affected by retinitis pigmentosa (RP), which predominantly affects retinal photoreceptor cells; secondary retinal neurons are relatively spared. Most RP patients will progress to near-total blindness. Several groups have developed variations of retinal prostheses for epiretinal or subretinal placement. These prostheses can help replace the function of the photoreceptors and stimulate secondary retinal neurons to create a visual image.
The Argus II device (Second Sight Medical Products) is the only FDA-approved epiretinal implant at this time. In 2002, Argus I, the first iteration of the device, which had 16 microelectrodes, was tested on human subjects who were enrolled in a phase 1 clinical trial. In 2011, Argus II, the second-generation device, with 60 microelectrodes, was approved for use in Europe. In 2013, after being granted Humanitarian Device Exemption status based on the safety and visual function results in 30 patients, it was granted U.S. FDA approval.
To date, more than 350 patients worldwide have been implanted with the Argus II retinal prosthesis.
Device Mechanism
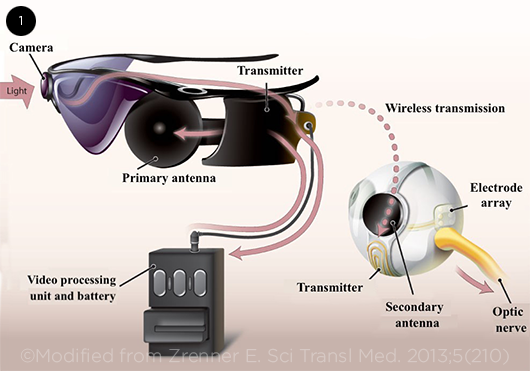
The Argus II system has three main components: a video camera attached to the frame of the patient’s glasses, a video processing unit (VPU) worn on a belt at the waist, and an epiretinal microelectrode-array implant connected to a secondary antenna (Fig. 1).
In real time, the VPU receives, processes, and converts the visual signal captured by the video camera into a brightness map. Data and power are wirelessly transmitted from the primary antenna, which is attached to the glasses, to the secondary antenna, which is sutured to the sclera in the lower temporal quadrant.1
The electronic data from the secondary antenna are then sent to the micro-electrode array, which is implanted on the patient’s retina. The array presents the brightness values from the video as pulse amplitudes on each of the 60 electrodes. This discrete signal is transmitted to the functioning secondary neurons, which help create a visual perception by processing and channeling the signal to the brain for final integration.
 |
|
ARGUS II. Components of the system.
|
Indications
Based on the inclusion criteria of a five-year clinical trial reported by da Cruz et al., the Argus II system is indicated for individuals with end-stage RP who are 25 years or older, with slight or no light perception bilaterally (>2.9 logMAR).1 The patient must have had functional form vision in the past to ensure intact optic nerve function and cortical processing. The worse-seeing eye is implanted with the Argus II prosthesis—and as the crystalline lens is removed during Argus II implantation, the patient can be pseudophakic or aphakic.
Patients’ willingness and ability to adhere to long-term low-vision rehabilitation, device training, and clinical follow-up after Argus II implantation are important factors to consider when assessing candidacy. Anatomic features that may prevent successful implantation, such as posterior staphyloma or axial length above or below the 20.5 mm to 26 mm range, and conditions that may hinder the device’s mechanism, such as impaired optic nerve function, exclude a patient from receiving the Argus II system.1
Surgical Technique
The surgical procedure for implanting the Argus II device is now well standardized. The sealed electronics enclosure and secondary antenna are attached to a silicone band that is placed around the globe and sutured onto the sclera like a scleral buckle. A vitrectomy is completed before introducing the microelectrode array into the eye through a pars plana sclerotomy. Finally, the microelectrode array is tacked to the retina over the macula.1,2
Benefits
It is challenging to test the effectiveness of the Argus II system because the patients who receive the device have little or no vision before implantation; therefore, common assessments such as visual field, visual acuity, and contrast sensitivity cannot fully quantify the real functional improvements in vision. It is important to note that it can take months for improvements to become apparent and stabilize because attaining adaptation to this new kind of vision requires a protracted learning process.
Instead, efficacy testing relies on assessing improvements in various aspects of daily living. Specifically, performance can be measured by tasks involving mobility and object discrimination and by questionnaires evaluating the patient’s opinion about the device’s effectiveness.
da Cruz et al. showed that, compared to the use of only residual vision, patients with the Argus II system activated were better at determining the direction of a moving object, performed better on an acuity task, and were more likely to locate a light shape on a dark background.1 Additional studies have demonstrated a clear improvement in the visual function of patients when using the device. Some patients with baseline minimal light perception were able to perceive hand motions and count fingers after activation of the device.3 Furthermore, those who most benefited were patients whose visual impairments were hindering their quality of life; after being implanted with the Argus II device, they reported considerable and sustained improvement in their quality of life.4
Long-Term Outcomes
No eyes were lost, and no patient’s residual vision was damaged in the five-year clinical trial.1 However, two of the 30 Argus II implants failed approximately four years after implantation due to a breakdown in the telemetry link between the primary and secondary antennae. The malfunction was thought to be caused by progressive exposure of the secondary antenna as a result of conjunctival erosion. In order to avoid this problem, Second Sight has modified the surgical procedure to cover the electronics case and sclerotomy site with processed human pericardium.2
In a separate study comparing the pre- and postoperative ocular coherence tomography images of 20 eyes, 50% were found to have developed a fibrosis-like tissue between the electrode array and surface of the retina.5 In the majority of patients, the fibrosis advanced to retinoschisis; however, no change was noted in visual function.
Based on current and previously published studies, the Argus II system appears to be relatively safe, with a safety profile comparable to other ocular implants such as glaucoma drainage devices. Three postapproval studies sponsored by Second Sight in the United States (NCT01860092), France (NCT02303288), and Germany/Italy (NCT01490827) continue to monitor long-term outcomes.
Cost
Device pricing in countries where Argus II is approved has ranged from $115,000 to $150,000 (U.S. dollars). The price of the device does not include the cost of medical and surgical interventions, training, or lengthy visual rehabilitation. However, despite the high initial outlay, a study evaluating the cost of the Argus II device demonstrated that it was a cost-effective intervention when compared to the standard care for RP.6 In the United States, Medicare carriers and most commercial payers have agreed to cover the cost of the Argus II for patients who are blind from end-stage RP, including evaluation, surgery, and rehabilitation.
Alternatives
In Europe, the IRIS II (Pixium Vision) and Retina Implant Alpha II AMS (Retina Implant AG), as well as the Argus II, are approved for use.
Like the Argus II, the IRIS II uses a VPU. However, the subretinally placed Alpha AMS uses a photodiode array that can simultaneously detect light and transfer a charge to the inner retina. Because of its subretinal placement, power supply with subdermal wires, and occipital connector, surgery is more technically challenging and takes longer for the Alpha AMS than for the Argus II.7 Initial results have shown that the Alpha II AMS implant is able to provide functional improvements such as identifying household objects and outdoor orientation in patients with RP who have residual inner retinal function.8 To facilitate direct comparison among various retinal implants, standardized assessment practices tailored to individuals with very low vision are needed.
Future Improvements
In its current form, the Argus II system can be improved by utilizing the modularity of its camera unit—camera modules with thermal sensitivity, depth selection, and zoom function are being used for specific environments, and they may soon be available to current Argus II implantees. Increasing the density and number of microelectrodes could also improve the functioning of the device because vision restoration is theoretically correlated with the number of microelectrodes.9
Alternatively, the diseased eye could be bypassed entirely with implantation of a prosthesis in the visual cortex. Second Sight announced in May 2019 that it plans to accelerate development and commercialization of its Orion Visual Cortical Prosthesis System, a brain implant, while temporarily suspending production of the Argus II system.
Research and development of new devices capable of providing increased spatial resolution would allow for further improvements in real-world functional capacity and quality of life.
Conclusion
Retinal prosthetic devices offer hope to patients with RP by bypassing the function of lost photoreceptors. Although the Argus II system’s safety profile has been validated through long-term clinical trials, ophthalmologists must select the appropriate patients; in addition, patients considering implantation must fully understand that the Argus II system provides limited visual restoration. Before proceeding, both patients and their ophthalmologists must set realistic expectations for improvement in daily activities and understand the long-term commitment required for functional rehabilitation.
___________________________
1 da Cruz L et al., for the Argus II Study Group. Ophthalmology. 2016;123(10):2248-2254.
2 Delyfer M-N et al. Ophthalmol Retina. 2018;2(4):276-287.
3 Humayun MS et al., for the Argus II Study Group. Ophthalmology. 2012;119(4):779-788.
4 Duncan JL et al. Clin Exp Optom. 2017;100(2):144-150.
5 Rizzo S et al. JAMA Ophthalmol. 2019;137(3):272-278.
6 Vaidya A et al. BMC Ophthalmol. 2014;14:49.
7 Lam BL, Grigori NZ. JAMA Ophthalmol. 2019;137(8):903-904.
8 Edwards TL et al. Ophthalmology. 2018;125(3):432-443
9 Chader GJ et al. Prog Brain Res. 2009;175:317-332.
___________________________
Mr. Bajaj is a fourth-year medical student at the Johns Hopkins School of Medicine in Baltimore. Dr. Dagnelie is associate professor of ophthalmology at the Johns Hopkins University School of Medicine and associate director of the Lions Vision Research and Rehabilitation Center at the Wilmer Eye Institute. Dr. Handa is chief of the Retina Division and the Robert Bond Welch Professor of Ophthalmology at Wilmer Eye Institute. Dr. Scott is associate professor of ophthalmology at Wilmer Eye Institute. Financial disclosures—Mr. Bajaj and Dr. Scott: None. Dr. Dagnelie: Second Sight; C,S. Dr. Handa: Second Sight; S.
See the disclosure key at www.aao.org/eyenet/disclosures.