By Matthew M. Rolain, BS, Matthew G.J. Trese, DO, MA, David M. Rooney, MD, Anant Krishnan, MD, Lori Stec, MD, and Robert J. Granadier, MD
Edited By: Ingrid U. Scott, MD, MPH
Download PDF
Poindexter Peterson,* an 84-year-old man, had been diagnosed with herpes zoster ophthalmicus (HZO). He had a vesicular rash over his left eye and the left side of his face, and he was prescribed a one-week course of oral antiviral medication. His rash and swelling almost completely resolved, but the pain persisted. One month after the initial diagnosis, no longer able to bear the intractable pain around his left eye, and unsure why his left eyelid was still droopy, Mr. Peterson went to the emergency department.
The Presentation
Medical history. Mr. Peterson had an extensive past medical history, including hypertension, chronic obstructive pulmonary disease, myasthenia gravis (which was in remission), obstructive sleep apnea, and multiple skin cancers.
Medications. He was taking albuterol, meclizine, metoprolol, and enalapril.
Ophthalmic history. His ophthalmic history included open-angle glaucoma treated with latanoprost and cataract extraction with implantation of a posterior chamber IOL in both eyes.
The Exam
Vision exam. On initial examination, Mr. Peterson’s visual acuity (VA) was 20/20 in his right eye and 20/60 in his left. Intraocular pressure (IOP) was 21 mm Hg in the right eye and 34 mm Hg in the left. Pupils were reactive without relative afferent pupillary defect (rAPD). Color vision, tested with Ishihara plates, was intact in both eyes.
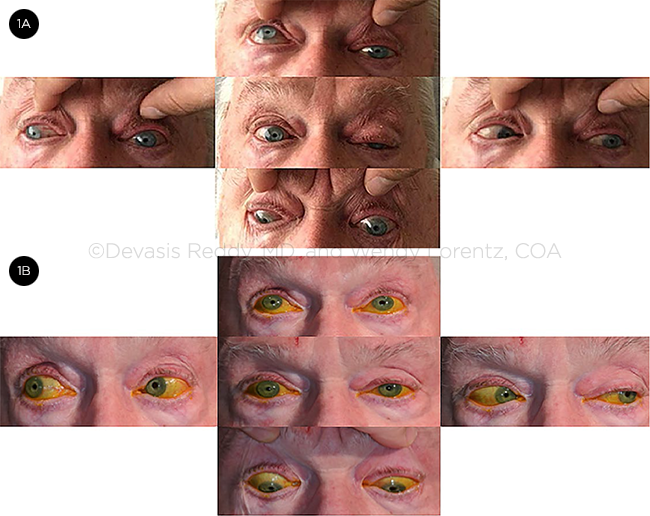
Extraocular movement. Extraocular movements (EOM) demonstrated significant limitation of ductions in all directions of gaze in the left eye (Fig. 1A). The remainder of the cranial nerve examination was unremarkable.
External exam. We noted complete ptosis, 2 mm of proptosis, and moderate injection in the left eye.
At the slit lamp. Slit-lamp biomicroscopy of the left eye revealed punctate epithelial keratopathy without dendritiform lesions, moderate central corneal stromal haze, and a deep and quiet anterior chamber.
Funduscopic exam. We noted a moderately enlarged cup-to-disc ratio, but the exam was otherwise normal.
First thoughts. The EOM limitations demonstrated involvement of multiple cranial nerves, bringing to mind two possible lesion locations: the orbital apex and the cavernous sinus. Because of the absence of an rAPD, we localized the lesion to the cavernous sinus and obtained emergent neuroimaging to confirm our suspicion. In addition to a mass lesion, our differential included Tolosa-Hunt syndrome, a painful ophthalmoplegia secondary to granulomatous inflammation of the cavernous sinus. However, given the association between HZO and cavernous sinus/superior orbital fissure syndrome,1 and the fact that the patient’s pain was consistent with postherpetic neuralgia in the distribution of the ophthalmic division of the trigeminal nerve (V1), we believed his current presentation was most likely related to HZO.
 |
|
EOM TEST. He showed decreased ductions in all directions of gaze upon presentation in the left eye (1A), with improvement on follow-up exam (1B).
|
Testing and Final Diagnosis
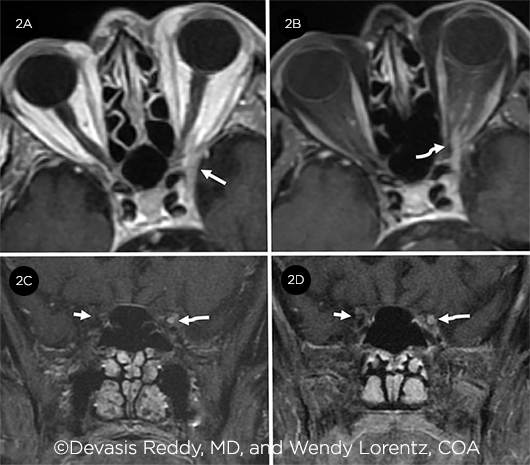
Magnetic resonance imaging (MRI) of the head and orbits with and without gadolinium demonstrated asymmetric thickening and enhancement of the left cavernous sinus, superior orbital fissure, and oculomotor nerve as it tracked from the cavernous sinus to the orbital apex. In addition, there was concern for an early septic thrombophlebitis (Figs. 2A and 2B). The patient was diagnosed with cavernous sinus syndrome secondary to chronic HZO. Mr. Peterson was admitted and received intravenous acyclovir and methylprednisolone. After five days of therapy, he was transitioned to oral valacyclovir and prednisone. Betaxolol was also initiated for IOP control in his left eye.
 |
|
IMAGING. Axial postcontrast T1–weighted image (2A) reveals asymmetrically thickened left cavernous sinus (arrow). Fat-saturated image (2B) also demonstrates a thickened and enhancing left third nerve (curved arrow) extending from orbit into cavernous sinus. Coronal fat-saturated T1 postcontrast image (2C) displays the difference between the abnormally thickened and enhancing left third nerve (curved arrow) from the right side (short arrow) where the normal nerve is difficult to even identify. Follow-up image (2D) after two months of intensive steroid and antiviral treatment reveals persistent thickened and enhancing left third nerve (curved arrow) with mild improvement in the thickened left cavernous sinus.
|
Follow-Up
Two months later, Mr. Peterson returned for a follow-up visit. VA was stable at 20/25 in his right eye and 20/60 in his left. IOP was 19 mm Hg bilaterally on ocular hypotensive therapy. Ptosis and duction deficits had significantly improved, but minimal limitations of supraduction and mild ptosis of the left eye remained (Fig. 1B). The patient was orthophoric in primary gaze and reported no diplopia.
Despite significant improvement in the ophthalmic exam, Mr. Peterson mentioned that he was feeling slightly off. He stated that he was confused and reported gait instability that had persisted for several hours. Confrontation visual fields revealed a new right homonymous hemianopsia.
Urgent neuroimaging identified an occlusion of the left posterior cerebral artery with associated acute/subacute ischemia of the left occipital and temporal lobes. Despite dramatic clinical improvement of the patient’s initial symptoms of ptosis and ophthalmoplegia, repeat MRI showed only slight improvement in the left cavernous sinus thickening and continued abnormal enhancement of the left oculomotor nerve (Figs. 2C and 2D).
Discussion
There are approximately 1 million cases of herpes zoster each year in the United States, of which HZO accounts for 10%-20%.2 HZO occurs when reactivation of varicella zoster virus (VZV) involves the V1 distribution. Ophthalmic complications of HZO can involve any of the ocular tissues, the orbit, and—in rare instances—the central nervous system (CNS).2 Resolution of the acute phase of HZO does not translate into remission, as evidenced by a significant number of patients who suffer a chronic/ recurrent disease course. Involvement of the orbital apex, cavernous sinus, and/or CNS is rare but can result in significant morbidity—and even mortality—if not addressed promptly. Neuroimaging is an important diagnostic tool, and its utility is optimized by localization as directed by comprehensive ophthalmologic examination. This case serves as a reminder that the constellation of ptosis and multiple cranial neuropathies without rAPD localizes the lesion to the cavernous sinus/superior orbital fissure.
The pathophysiologic mechanism underlying HZO-associated cranial neuropathies is unknown. Histologic studies that have attempted to elucidate an underlying etiology have focused on either direct infection of the cranial nerves or inflammatory changes resulting in vascular and neurologic compromise.3 While there is no agreed-upon consensus on treatment, dual therapy with antivirals and steroids, which aim to treat the reactivated virus and mitigate the inflammatory damage, is the mainstay of therapeutic intervention.
Finally, our patient—with multiple known risk factors (i.e., age, hypertension, and obstructive sleep apnea) for cerebrovascular accident (CVA)—developed a posterior circulation stroke shortly after VZV reactivation. In a recent meta-analysis, HZO was associated with an increased risk of stroke within three months to one year of HZO re-activation.4 The mechanism that underlies this association is unknown. One possible explanation for the relationship between HZO and CVA was proposed by Grose and Adams.5 Upon viral reactivation within the dorsal root ganglia of the trigeminal nerves, there is anterograde transmission of the virus within the nerves. These nerves terminate within the adventitia of nearby cerebral vasculature, specifically the internal carotid artery and Circle of Willis. Grose and Adams postulate that viral replication may extend to this vasculature, resulting in an occlusive inflammatory vasculitis. Support for this theory in large part stems from the giant cell arteritis literature, where VZV has similarly been implicated as a contributing factor for occlusive vasculitic disease.6
Take-Home Points
This case underscores the importance of complete ophthalmologic evaluation in cases of HZO, as our patient’s cavernous sinus/superior orbital fissure syndrome went undiagnosed for weeks because ptosis masked the recognition of ophthalmoplegia and diplopia.
Further research into the pathophysiologic mechanisms underlying HZO involving the cavernous sinus/superior orbital fissure should allow for optimized treatment protocols. And because VZV is associated with significant morbidity, this case should remind us of our role in advising patients to discuss the potential benefits of shingles vaccination with their primary care physician.
___________________________
*Patient name is fictitious.
___________________________
1 Kedar et al. J Neuroophthalmol. 2019;39(2):220-231.
2 Liesegang TJ. Ophthalmology. 2008;115(2 Suppl):S3-12.
3 Naumann G et al. Am J Ophthalmol. 1968;65(4):533-541.
4 Erskine N et al. PLoS One. 2017;12(7):e0181565.
5 Gilden D et al. Neurology. 2015;84(19):1948-1955.
6 Grose C, Adams HP. Expert Rev Anti Infect Ther. 2014;12(5):527-530.
___________________________
Mr. Rolain is a medical student at Wayne State University School of Medicine in Detroit. Dr. Rooney is a glaucoma fellow at the University of Pittsburgh Medical Center. Dr. Krishnan is a radiologist at Beaumont Hospital in Royal Oak, Mich. Dr. Trese is an ophthalmology resident, Dr. Stec is a comprehensive ophthalmologist, and Dr. Granadier is a neuro-ophthalmologist; all three are at Beaumont Eye Institute in Royal Oak, Mich. Financial disclosures: None.