By James P. Winebrake, BS, Owen J. Drinkwater, BS, BA, Ashley R. Brissette, MD, MSC, and Christopher E. Starr, MD
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
In 2007, the Tear Film and Ocular Surface Society (TFOS) published the Report of the TFOS International Dry Eye Workshop, later known as TFOS DEWS. The original report expanded the scope of dry eye disease (DED), and since that time, the number of publications on the topic has roughly doubled.
A decade later, the highly anticipated TFOS DEWS II was released online in The Ocular Surface journal.1-9 The full report, comprising more than 350 pages, tackles the published literature on DED since the original TFOS DEWS. This article briefly summarizes key points from each section of the report and aims to increase awareness of the findings and recommendations from this landmark publication.
Definition and Classification1
TFOS DEWS II defines dry eye disease as the following: “Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”
Establishing an appropriate definition for DED is extremely important because it serves to guide diagnostic methodologies and treatment modalities.
Changes to definition. Notable differences from the 2007 TFOS DEWS definition include the recognition of the loss of tear film homeostasis as a key characteristic of DED as well as the acknowledgment of neurosensory abnormalities as an etiologic factor.
Aqueous deficient or evaporative? Identifying the primary DED subtype (aqueous deficient or evaporative) remains important for classification. However, the consensus of TFOS DEWS II is that these subtypes are part of a spectrum of disease, rather than being distinct pathophysiological entities. Determining the predominant underlying etiology can be useful in directing primary treatment strategies.
Sex, Gender, and Hormones2
Sex versus gender. Although interrelated, sex and gender are distinct concepts that should be considered separately in the medical literature. The former is based on biological characteristics, the latter on societal constructs and definitions (including self-definition).
Sex-specific factors. Female sex has been identified as a risk factor for the development of DED across many studies. Some of the contributors to the higher prevalence of DED in women may include synthesis and interaction of tear film components; balance of sex steroids, including androgen deficiency; and even anatomic differences in structures including the meibomian glands and lacrimal apparatus.
Women also reported higher rates of depression, greater impact of DED on quality of life, and more negative treatment side effects, with a longer time required for improvement.
More study needed. The TFOS DEWS II authors noted that the terms sex and gender were used interchangeably in many studies, making it difficult to tease apart factors related to biology versus societal identity, such as gender differences in health care utilization and expression of pain. Moreover, the effects of sex-, gender-, and hormone-based factors on DED are multifaceted, meriting further investigation and mindfulness in the clinical setting.
 |
|
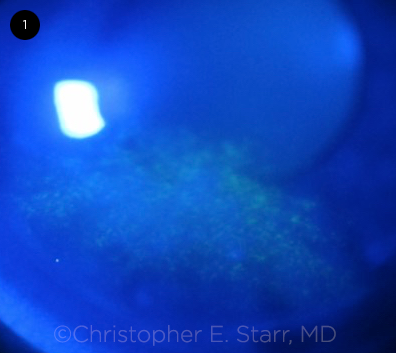
DED. Inferior corneal staining with fluorescein in dry eye disease.
|
Epidemiology3
Wide-ranging estimates. Assessment of large-scale DED prevalence and incidence has been hindered by inconsistencies in the definition and diagnostic criteria among prior studies. When the diagnosis is based on symptoms (with or without signs), meta-analysis yields prevalence values ranging from 5% to 50%; when signs alone are used, the prevalence is as high as 75% in certain cohorts.
Selected findings. Large-scale studies have determined that increasing age, female sex, and Asian race are all associated with higher DED prevalence. A small number of studies have found a high prevalence of symptoms among children and young adults. It has been postulated that increasing use of digital devices may be a risk factor in this population, although more studies are needed to clarify the relationship.
Areas for future research. There is a paucity of research from the Southern Hemisphere, so the possible effects of any regional and cultural differences have gone largely unstudied. In addition, as few studies have been published on the natural history of DED, this is another topic that could benefit from future research.
Tear Film4
The tear film overlies the conjunctiva and cornea, with a precorneal thickness of approximately 2.0 to 5.5 μm.
Layers of the tear film. The tear film has traditionally been described as a 3-layered structure consisting of a mucin layer, an aqueous layer, and a lipid layer. In contrast, the TFOS DEWS II depicts the tear film as a 2-layered interactive structure composed of a mucoaqueous layer and a lipid layer.
The mucoaqueous layer serves to reduce friction and provide hydration to the ocular surface, while the lipid layer serves to decrease surface tension and minimize evaporation and tear film instability. Each blink drives capillary movement and upward drift of the lipid and mucoaqueous layers, which contribute to proper tear distribution across the corneal and conjunctival surfaces.
Osmolarity and other factors. The tear film osmolarity is an extremely important measure of DED, and hyperosmolarity of either eye (> 308 mOsm/L) or an intereye difference > 8 mOsm/L is suggestive of tear film instability.
In addition to currently available assessment modalities, including tear osmolarity, matrix metalloproteinase-9, and lipid layer interferometry, many other tear components have been identified as possible biomarkers for DED and will be of interest in future studies.
Lastly, contact lens wear, systemic hormone levels, and environmental conditions (humidity and temperature) also affect tear film composition and stability.
Pain and Sensation5
Awareness and understanding of the role of the corneal nerves in DED have increased substantially since the original TFOS DEWS report.
Neuropathic pain, or neuralgia, describes a lesion of the somatosensory system, which may lead to the perception of pain in the absence of clinical objective signs of tissue damage but should not be diagnosed as classic DED. When diagnosed as a primary condition, keratoneuralgia is often described as “pain without stain.” However, chronic or persistent DED that damages the corneal nerve fibers—which are prone to damage due to their high density and superficial location within the cornea—may lead to somatosensory and central nervous system sensitization and subsequent symptoms of neuropathic pain in the setting of decreased corneal sensation.
Corneal sensitivity. At the highly innervated cornea, sensory fibers of the trigeminal nerve respond to a variety of stimuli—mechanical, chemical, and thermal—that can induce pain symptoms of DED. In most studies, patients with DED—aqueous deficient DED in particular—demonstrate reduced corneal sensation to mechanical stimuli from an esthesiometer, which is thought to be due to corneal epithelial damage. In DED, the polymodal and mechano-nociceptors undergo sensitization in response to long-standing inflammation. The time course is also important; persistent damage to the corneal epithelium likely decreases tissue sensitivity, whereas long-term damage to underlying nervous tissue is thought to contribute to abnormal neural activity and subsequent neuropathic pain sensation.
Additionally, abnormally heightened activity is seen in cold thermoreceptors, which act by detecting temperature and changes in tear film osmolarity. Their activation leads to an increase in basal tear production and blink reflex.
Neural imaging. In vivo confocal microscopy has been used to image corneal nerves and inflammatory cells in the setting of DED, revealing characteristic neural tortuosity, reflectivity, and beading. Less well established are changes in neural density patterns, potentially accounting for the heterogeneity of therapeutic response and the discrepancies between observed signs and reported symptoms of assumed DED.
Clinical assessment. In the clinical setting, questionnaires assessing DED pain and sensation usually elicit foreign body sensation and light sensitivity, but responses are otherwise often quite varied.
Pathophysiology6
The vicious circle. Hyperosmolarity at the ocular surface initiates an inflammatory sequence leading to damage of epithelial cells, goblet cells, and the glycocalyx, observed clinically as punctate epitheliopathy and tear film instability and breakup. Described by the TFOS DEWS II as a “vicious circle” of inflammation, this process is the common final pathway for all forms of DED.
In a self-perpetuating cycle, evaporative losses cause frictional damage to the lid and ocular surface, exacerbating hyperosmolarity and inducing the aforementioned inflammatory sequence yet again. The loss of homeostasis of the ocular surface is the key pathophysiologic change that defines DED.
Evaporative versus aqueous-deficient dry eye. Environmental conditions, contact lens wear, decreased blink frequency, and other factors predispose to ocular surface–related evaporative dry eye (EDE) in regions of tear film instability, whereas meibomian gland dysfunction–related EDE involves widespread deficiency of the tear film lipid layer.
Aqueous-deficient dry eye is caused by reduced lacrimal secretion. It may be attributable to advanced age, systemic drug use, sensory reflex block, or inflammatory infiltration (as in Sjögren syndrome) or to lacrimal duct obstruction observed in cicatricial conjunctival disease and other pathology.
Although the primary cause of DED may vary, all forms enter the final common vicious circle of inflammation leading to clinical signs and symptoms of DED. Determining the primary cause of DED is crucial to developing an effective treatment protocol.
Iatrogenic Dry Eye7
DED can develop secondary to various medical or surgical interventions.
Medications. Oral medications (including antihypertensives and antihistamines) are among the most common causes of iatrogenic DED. Management scenarios include stopping the offending medication, lowering the dose, or, alternatively, treating the iatrogenic DED.
Eyedrops for glaucoma (and other topical drops), especially those containing preservatives, have also been shown to contribute to ocular surface disease. Preservative-free or lower-dose drop formulations and/or laser or surgical procedures to manage glaucoma are treatment options. Lubricant drops may also be helpful.
Surgery. Corneal refractive and cataract surgery are among the major causes of iatrogenic DED. Either postoperative development of DED or exacerbation of preexisting DED can lead to decreased patient satisfaction and poor visual outcomes. Thus, DED should be diagnosed and managed preoperatively in order to optimize the ocular surface and minimize DED-related postoperative complications.
Diagnostic Methodology8
It is well known that the signs and symptoms of DED are poorly correlated. As such, there is no single gold-standard diagnostic marker for this complex condition.
Patient questionnaires. A variety of validated questionnaires are available for assessing the symptoms of DED, and TFOS DEWS II presents a diagnostic strategy for their implementation. Screening questions are useful at the beginning of the assessment in order to rule out DED secondary to other conditions. DED questionnaires should then be administered to determine subjective severity of symptoms and their effect on quality-of-life measures.
Signs. In addition to symptoms, the clinical protocol for DED diagnosis requires the presence of at least one abnormal homeostatic marker. Positive markers include decreased tear breakup time, tear film hyperosmolarity, or ocular surface staining (corneal or conjunctival, as demonstrated with lissamine green or fluorescein; Fig. 1).
Meibomian gland features and tear meniscus assessment can help to classify the predominant DED subtype and severity and to direct initial management.
Differential diagnosis. The differential for DED-like symptoms is extensive and includes conjunctivitis, blepharitis, Sjögren syndrome, infection, and lid-related disease. Implementing a repeatable, step-by-step diagnostic process is essential in diagnosing DED and excluding other ocular surface conditions that may mimic DED symptoms.
Management and Therapy9
TFOS DEWS II presents an evidence- based, multistaged management algorithm in order to determine the most appropriate DED treatment for each patient, based on subjective and objective severity measurements.
A stepwise approach. Step 1 (of 4) includes patient education; environmental modifications; dietary recommendations; and home treatment with lid hygiene, warm compresses, and lubricating eye drops.
Step 2 comprises management with prescription medications, including topical steroids, cyclosporine, leucocyte function–associated antigen-l antagonists, secretagogues, and topical or oral antibiotics. This step also includes placement of punctal plugs and other minor in-office procedures such as meibomian gland expression and intense pulsed light application.
Step 3 includes oral secretagogues, bandage or scleral contact lenses, and autologous serum eyedrops.
Step 4 is reserved for refractory DED that may require long-term topical corticosteroids, amniotic membrane grafting, or surgical intervention such as permanent punctal occlusion, tarsorrhaphy, and other eyelid procedures.
Further Information
This summary can only touch on highlights from the workshop. For greater detail, as well as the reports and methodology of each of the subcommittees, see the full TFOS DEWS II document at www.tearfilm.org (open access) or in The Ocular Surface, July 2017 issue.
___________________________
1 Craig JP et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283.
2 Sullivan DA et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15(3):284-333.
3 Stapleton F et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-365.
4 Willcox MD et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366-403.
5 Belmonte C et al. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15(3):404-437.
6 Bron AJ et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.
7 Gomes JA et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511-538.
8 Wolffsohn JS et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-574.
9 Jones L et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628.
___________________________
Mr. Winebrake and Mr. Drinkwater are medical students, Dr. Brissette is an assistant professor of ophthalmology, and Dr. Starr is an associate professor of ophthalmology; all are at Weill Cornell Medicine in New York City. Relevant financial disclosures. Dr. Brissette: Allergan, C. Dr. Starr: Allergan, C; Alcon/Novartis, C; Bausch & Lomb, C; Rapid Pathogen Screening, C; Shire, C; TearLab, C. Mr. Winebrake and Mr. Drinkwater: None.
See the disclosure key at www.aao.org/eyenet/disclosures.
More at the Meeting
Attend Ocular Surface Disease: What You May Be Missing (Sym23). When: Monday, Nov. 13, 8:30-10:00 a.m. Where: New Orleans Theatre C. Access: Free.
|