Download PDF
Infusion of cytotoxic T-cells are offering hope to patients who are refractory to or intolerant of current therapies for cytomegalovirus (CMV) retinitis. The novel therapy involves CMV-specific cytotoxic T lymphocytes (CTLs)—and despite the immune privilege status of the eye, infusion of these cells led to resolution of CMV retinitis in 90% of treated eyes in a small study.1
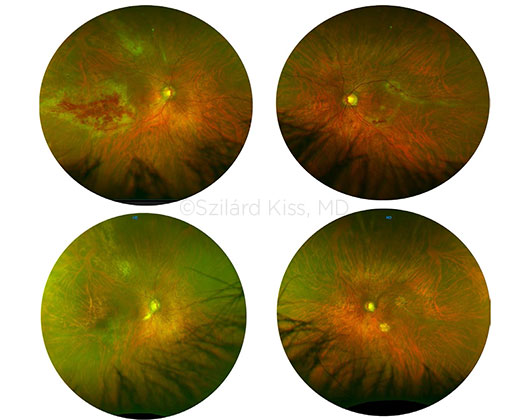 |
BILATERAL DISEASE. This patient had HIV, active lymphoma, and CMV retinitis. Despite receiving twice-weekly intravitreal injections and systemic treatment with antivirals for the retinitis, the disease was progressively worsening and threatening his vision. (Top) Active CMV retinitis at presentation. Polymerase chain reaction (PCR) results showed 1.5 × 10e7 CMV viral particles in the right eye and the UL97 mutation. Treatment involved four rounds of CTL infusions. Oral medication for CMV retinitis was discontinued because of severe side effects; intravitreal injections also were discontinued. (Bottom) Nearly three years following the CTL infusions, no signs of disease are evident in either eye. A follow-up PCR for CMV also was negative. The patient continued to be severely immunocompromised due to his ongoing treatment for recurrent lymphoma.
|
Adoptive cell transfer. The treatment was originally developed for cancer patients, said Szilárd Kiss, MD, at Weill Cornell Medical College in New York City. A team of researchers at Memorial Sloan-Kettering Cancer Center developed a library of CMV-specific CTLs from a pool of T-cell donors, and each CMV CTL line was genotyped for the human leukocyte antigen system.
“The broad concept is that you and I have T cells that recognize CMV, and we can take those T cells and bank them,” Dr. Kiss said. When a patient comes in with resistant CMV, “we can take the T cells that match [the patient]—and it doesn’t even have to be an exact match.” The treatment essentially re-establishes the patient’s immune cell activity against CMV, Dr. Kiss said. “Your immune system is trained to fight off CMV even after the infused donor cells are gone.”
Study specifics. For this study, Dr. Kiss and his colleagues evaluated the ocular outcomes of a subset of 27 patients who were treated for CMV retinitis at Weill Cornell Medical College. The subset comprised seven patients (10 eyes) who received infusions of CMV-specific CTLs. All seven were men; their average age was 53 years (range, 26-68 years). All had undergone treatment with systemic and intravitreal antiviral medications, and their average best-corrected visual acuity (BCVA) at baseline was 20/91 (range, 20/20 to counting fingers).
During an average follow-up of 30 months (range, 6-76 months), the CMV retinitis resolved completely in nine of the 10 eyes, even in light of continued immunosuppression of the patients. Mean BCVA at final follow-up was 20/78 (range, 20/20 to no light perception). No immune recovery uveitis was noted.
Potential recipients. During the HIV epidemic, CMV retinitis was a risk for many AIDS patients, Dr. Kiss noted. “Now, the face of CMV retinitis is transplant patients, who are facing indefinite immune suppression, and cancer patients, specifically those who’ve undergone bone marrow transplantation.”
Standard care for CMV retinitis primarily involves antiviral therapy with agents such as ganciclovir and foscarnet. However, “there are refractory cases that are resistant to standard therapy,” said Dr. Kiss. In particular, certain mutations in the virus render patients CMV resistant. Moreover, as he noted, “some patients are intolerant to standard care, which may require bone marrow–suppressing systemic treatment.”
For these patients, who have no other options, this therapy is a game-changer, Dr. Kiss said. He offered the example of a patient with the relevant antiviral resistance mutations. “After four infusions, he’s cured—although the patient’s immune system is still compromised, because he had a bone marrow transplant.”
Next steps. “We’ve received inquiries from around the world for patients who have CMV retinitis—and even CMV esophagitis that is ravaging their gut,” Dr. Kiss said. But availability is an issue, as patients currently need to come to New York for treatment. “Memorial Sloan-Kettering is trying to figure out how to make this therapy available to more patients. The hope is that a commercial partner will step up,” he said.
And as Dr. Kiss and his colleagues noted, additional studies are needed to evaluate the treatment’s safety and efficacy in a larger group of patients and to clarify whether it is best used as monotherapy or as an adjunctive treatment.
—Jean Shaw
___________________________
1 Gupta MP et al. Ophthalmol Retina. 2021;5(9): in press.
___________________________
Relevant financial disclosures—Dr. Kiss: Intellectual property related to CTL treatment assigned to Cornell University.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Binenbaum Luminopia: O; X-Biomedical: O.
Dr. Kiss Adverum: C,O; Alcon: C,S; Allergan: S; Atara Biotherapeutics: P; Cornell University: P; Fortress Bio: O; Genentech/Roche: C,S; Novartis: C,S; Optos: C,S; Regeneron: S; Regenxbio: O. Intellectual property related to CTL treatment assigned to Cornell University.
Dr. Lee Klorfine Family Endowed Chair: S; National Institute on Aging: S; Research to Prevent Blindness: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review